
Design of Semiconducting Gas Sensors for Room-Temperature Operation
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(http://creativecommons.org/licenses/bync/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Gas sensors that operate at room temperature have been extensively studied because of sensor stability, lift time, and power consumption. To design effective room-temperature gas sensors, various nanostructures, such as nanoparticles, nanotubes, nanodomes, or nanofibers, are utilized because of their large-surface-to-volume ratio and unique surface properties. In addition, two-dimensional materials, including MoS2, SnS2, WS2, and MoSe, and ultraviolet-activated methods have been studied to develop ideal room-temperature gas sensors. Herein, a brief overview of state-of-the-art research on room-temperature gas sensors and their sensing properties, including nanostructured materials, two-dimensional materials, the ultraviolet-activated method, and ionic-activated gas sensors, is provided.
Keywords:
Gas sensor, Semiconductor, Room-temperature operation, Principle, Review1. INTRODUCTION
A gas sensor is defined as a device that detects the presence of reactive gases and monitors the gas concentration. In addition, it offers real-time information on the presence of specific gases, which is effective in various fields, including the indoor air quality, medical, industry, and military fields [1,2]. The types of the gas sensor have been classified according to the detection method, such as Schottky diode, acoustic-wave, catalytic combustion, electrochemical, optical, and chemiresistive types [3-6].
For Schottky diode sensors, target gases are dissociated by the catalytic metal electrodes, followed by diffusion of the atomic species to the semiconductor interfaces between the electrode and sensing materials, where the effective barrier height changes upon exposure to target gases.
Acoustic-wave sensors utilize chemically selective sensing layers coated by piezoelectric substrates. When the sensing elements are exposed to the target gas, the adsorption of gaseous species on the sensing layer acts as a mass, resulting in a decrease in a resonant frequency.
Optical gas sensors detect changes of optical parameters (including refractive index, reflection of light, absorption coefficient, and fluorescence scattering) in the presence of target gases.
For electrochemical gas sensors, two electrodes (working and counter electrodes) are separated by a thin layer of electrolytes. When target gases are introduced, an electrochemical reaction (oxidation or reaction) occurs at the working electrode, and the current is detected depending on the gas concentration.
The chemiresistive type is based on the changes in resistance upon exposure to target gases. When the gas is introduced to the sensor, chemical reactions occur on the surface of the sensing materials, resulting in a resistance change of the sensors.
Compared with other types, the chemiresistive sensor has been extensively studied because of its simple operation, cost effectiveness, and flexibilities that enable them to be applied to existing circuits. In particular, the potential of the chemiresistive type arises from the high sensitivity, minimized size, and compatibility with silicon-based technology, which enables this type to be suitable for developing portable instrumentation [7].
To design high-response and reversible chemiresistive gas sensors, a high operating temperature of 150–400°C is essentially required for adsorption and desorption of target gases on the surface [8,9]. However, the high-temperature operation significantly damages interconnected devices and brings the risk of ignition when detecting flammable or explosive gases [10,11]. Moreover, it requires continuous high power consumption to operate the sensors [12]. Hence, gas sensors that operate at room temperature have been extensively studied using a variety of approaches, including nanostructured materials, two-dimensional (2D) materials, the self-heating method, and the ultraviolet (UV)-assisted method [13,14].
In this review, the past developments of room-temperature gas sensors are summarized, including nanostructured materials, 2D materials, and the UV-assisted method, with a theoretical background of conventional chemiresistive gas sensors. In addition, the current progress of state-of-the-art ionic-activated gas sensors is outlined for ideal operation at room temperature.
2. THEORETICAL BACKGROUND
Semiconducting gas sensors are classified as n-type and p-type sensors depending on their majority charge carriers (electron or hole). Both n-type (SnO2, In2O3, WO3, and Fe2O3) and p-type (NiO, Co3O4, Cr2O3, and CuO) semiconductors adsorb oxygen molecules on the surface [15]. The adsorption mode is mainly influenced by operating temperature [16]. At a low temperature, the gas molecules are trapped in a physisorbed state on the surface of sensing materials through weak van der Waals forces (Fig. 1). This process is called physisorption, and the oxygen (O2) is physisorbed at a low temperature of less than 150°C, forming physisorbed oxygen (). This physisorbed molecule is always held at a greater distance than it would be if a chemical bone were formed, typically >4 bonding length [17]. As the temperature increases, the physisorbed molecules overcome the activation energy (ΔEact), leading to the formation of chemical bonds close to the surface. Therefore, the oxygen species are chemisorbed on the surface as a form of chemisorbed oxygen (O– or O2–). The possible reaction paths are as follows:
| (1) |
| (2) |
| (3) |
| (4) |
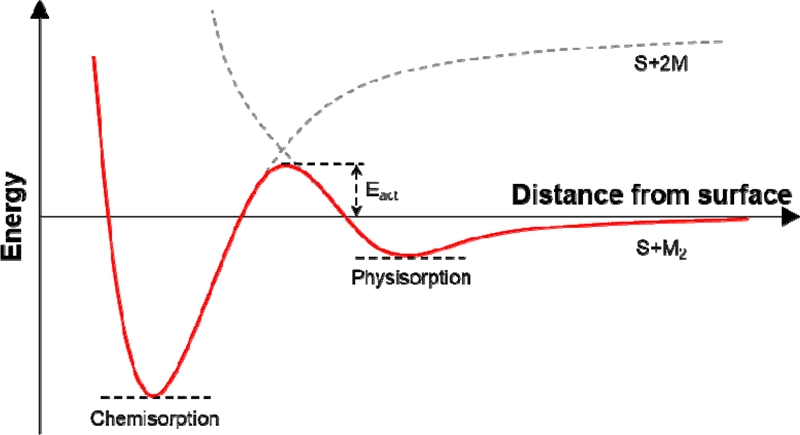
Potential energy diagram for the case of physisorption and chemisorption, where the S and M indicates the sensor surface and the target molecules, respectively.
For n-type semiconductors, the adsorption of oxygen species increases the sensor resistance by extracting electrons from the conduction band of the sensing materials, resulting in formation of a highly resistive depletion layer. In contrast, electron–hole pairs are generated depending on the temperature, leading to an increase in sensor conductivity. Therefore, the sensor resistance generally increases up to 300°C by the formation of the chemisorbed oxygen species. Then, it decreases with elevating of the operating temperature by the generated electron–hole pairs and desorption of oxygen species. When the gas sensor is exposed to reducing gases, such as CO, CH3COCH3, and NH3, it is oxidized by reacting with preadsorbed oxygen species on the surface, and the captured electrons are released back to the sensing materials. Therefore, the resistance is significantly decreased for the n-type sensors. For oxidizing gases, such as SO2, O3, and NO2, the electrons are extracted from the sensing materials by direct adsorption on the surface, leading to an increase in the sensor resistance.
| (5) |
| (6) |
In general, the maximum response to the reducing gas is observed at the temperature with the highest base resistance because of the trade-off relation between the adsorption and desorption rates of oxygen species according to the operating temperature. In contrast, oxidizing gas competitively adsorbs with the oxygen on the surface of sensing materials; thus, the maximum response is observed at a relatively low temperature of 100–200°C.
For the gas sensors to operate at room temperature, the target gas should adsorb on the surface of sensing materials, and this leads to modulation of electrical conductivity. However, these processes are restricted because of the insufficient reaction energy at room temperature. In addition, it is difficult to produce a large resistance change through physisorption. Therefore, there have been various attempts to design room-temperature gas sensors using nanostructured materials, 2D materials, the UV-assisted method, and ionic-activated method.
3. APPROACHES FOR ROOM-TEMPERATURE OPERATION
3.1 Nanostructured Materials
The interaction between gas molecules and materials mainly takes place on the surface of the semiconductor. Thus, the number of atoms residing at a material surface is critical for controlling the sensor performance. From this point of view, nanostructured materials composed of nanosized building blocks have demonstrated great potential for use as the sensing layers [13]. Because of the large surface-to-volume ratio, the nanostructured materials have a much larger portion of surface atoms than bulk atoms in comparison with bulk materials [18]. In addition, it has been reported that specific crystal facets with high surface reactivity improve the gas-sensing performance [19]. Research endeavors have demonstrated that a variety of materials show appealing features for room-temperature gas sensors. Bianchi et al. studied In2O3 microwire for room-temperature NO2 sensors [20]. In addition, Pan et al. demonstrated a ZnO nanowire-based gas sensor at room temperature [21]. Despite these extensive efforts, challenges remain, including poor response and incomplete recovery, because of the restricted modulation in electronic conductivity by insufficient reaction energy between the analytes and sensing materials.
2.2 Two-Dimensional Materials
To improve the gas-sensing performance further, 2D layered materials have been widely studied for room-temperature operation [22]. These 2D materials, such as MoS2, WS2, ReS2, and SnS2, are composed of an atomically thin-layered structure and show a large surface-to-volume ratio, good signal-to-noise ratio, and high chemical stability. In particular, an ultrathin-layered structure limits the current paths, and this is directly influenced by the surface response to target gases. Li et al. reported single-layer and multilayer (one to four layers) MoS2 for NO sensing at room temperature [23]. Fig. 2a shows an optical image of 2L MoS2 fabricated by an exfoliation method with a field effect transistor. The NO sensing properties are shown in Fig. 2b. With increasing NO concentration, the change in sensor current continuously increases. Further improving the room-temperature gas-sensing properties based on 2D materials, Kwon et al. proposed SnS2 nanograins on SiO2 nanorods [24]. The SiO2 nanorods were used as a hard template to maximize the surface-to-volume ratio and expose edge sites of SnS2 on the surface (Fig. 2c). As shown in Fig. 2d, the gas response to 10-ppm NO2 increased for the SnS2 distributed on thicker SiO2 nanorods, and SnS2 on SiO2 nanorods with 500-nm thickness exhibited the highest gas response of 701%. The exposed edge sites were reported to have contributed to the enhanced gas-sensing properties.
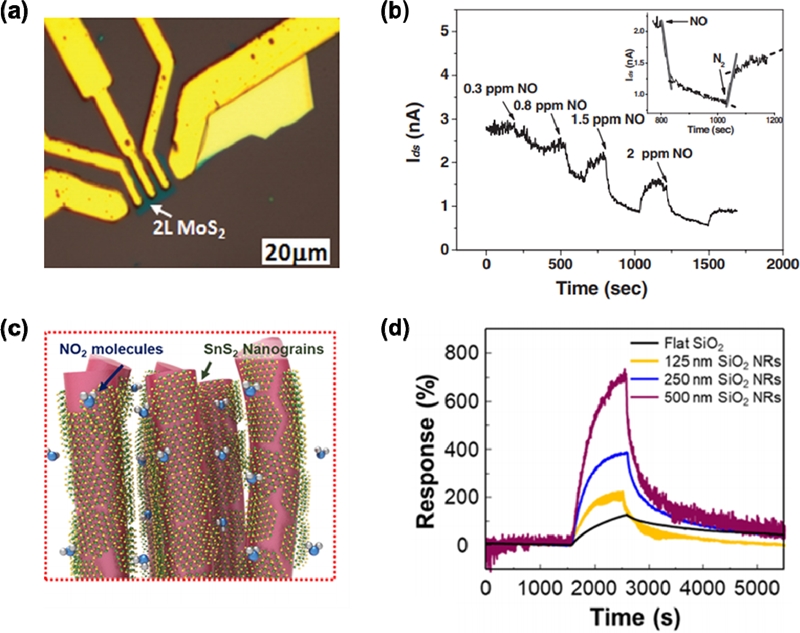
(a) Optical microscope image of a bilayer (2L) MoS2 film deposited onto Si/SiO2. (b) Current response to NO of the 2L MoS2 sensors [23]. (c) Schematic illustration of SnS2 nanograins on SiO2 nanorods. (d) Response transient of SnS2 on each different SiO2 nanorods template to 10 ppm NO2 at room temperature [24]. Reprinted with permission from [24]. Copyright (2019) American Chemical Society.
2.3 UV-Activation Method
As another approach, illuminating the sensing layer with UV photons is an interesting strategy to design the room-temperature gas sensors [25]. The energy of the UV photon is comparable to or larger than the bandgap of the sensing materials. When the UV light is introduced to the sensors, the material conductivity is enhanced by the generation of a photocurrent that increases the number of free carriers. This photoexcitation not only enhances the sensor response but also improves response and recovery speed. In a pioneering work, Fan et al. reported a UV-activated room-temperature gas-sensing mechanism [26]. The electrons generated by UV light promote the adsorption of oxygen species and form the photoinduced oxygen ions. These ions are highly reactive and responsible for the room-temperature gas response. Fig 3a shows responses of ZnO2 thin film to 100-ppm H2 upon illumination by UV light. Compared with other conditions, the nanoline under UV light shows a much higher response and fast recovery because of photoinduced oxygen ions. Kumar et al. reported UV-activated MoS2-based NO2 sensors for room-temperature operation (Fig. 3b) [27]. Upon illumination with UV light, the MoS2 sensor exhibited an enhancement in response, with an ultrafast response time of ~29 s and excellent recovery to NO2 (100 ppm) at room temperature (Fig. 3d). In addition, Park et al. reported NO2 response as a function of UV light intensities [28]. With increasing the intensity from 0 to 1.2 mW/cm2, the response to 5-ppm NO2 is continuously increased from 189% to 619% (Fig. 3c). However, the UV-activated method essentially requires a high power consumption to operate the UV generator, and this is not consistent with the purpose of developing room-temperature gas sensors.
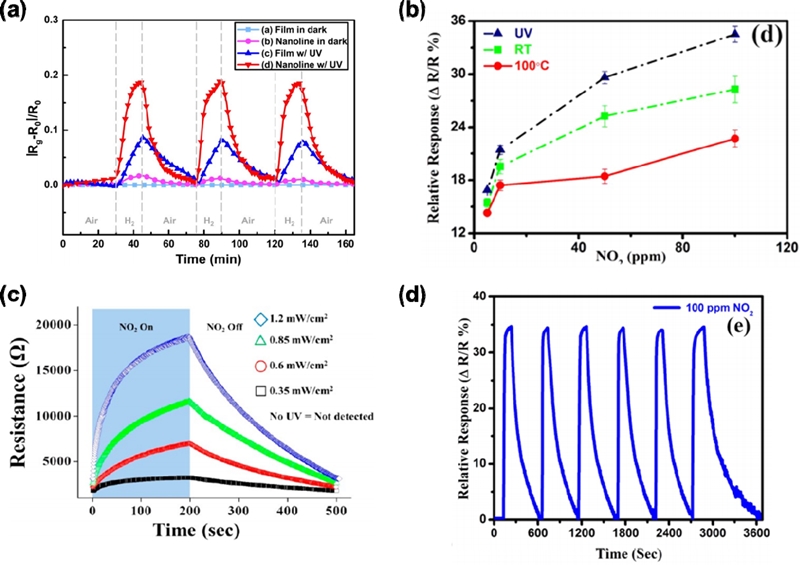
(a) Response of ZnO thin film and nanoline upon exposure UV light to 100 ppm H2 [26]. (b) Response of MoS2 to NO2 under room temperature, UV-activation, and 100 °C. (d) Cycle test to 100 ppm NO2 under UV light [27]. Reprinted with permission from [27]. Copyright (2017) American Chemical Society. (c) response of SnO2-core/ZnO-shell nanowires to NO2 gas at 5 ppm NO2 for different UV light illumination intensities [28]. Reprinted with permission from [24]. Copyright (2013) American Chemical Society.
2.4 Ionic-Activated Gas Sensors
Until now, the introduced room-temperature gas sensors were operated through an electronic conduction mechanism. To overcome the problem, a different kind of gas-sensing mechanism is needed for room-temperature gas sensors with high response and fast recovery. As mentioned, the conventional electronic conduction-based gas-sensing mechanism is limited at room temperature because of restricted reaction energy between the surface of the sensing materials and target gases. To overcome these drawbacks, Song et al. reported novel ionic-activated chemiresistive gas sensors for room-temperature operation [29]. The ionic-activated sensors operate by the ionic conduction induced by adsorbed water molecules on the surface of sensing materials under humid conditions. This ionic conduction was systematically investigated through in situ X-ray photoelectron spectroscopy, electrochemical impedance spectroscopy (EIS), and I–V characteristics. Fig. 4a shows the Nyquist plot based on EIS. The straight line above relative humidity (60%) indicates Warburg impedance generated by diffusion resistance, which is well characterized by the Grotthuss and vehicle mechanisms [30].
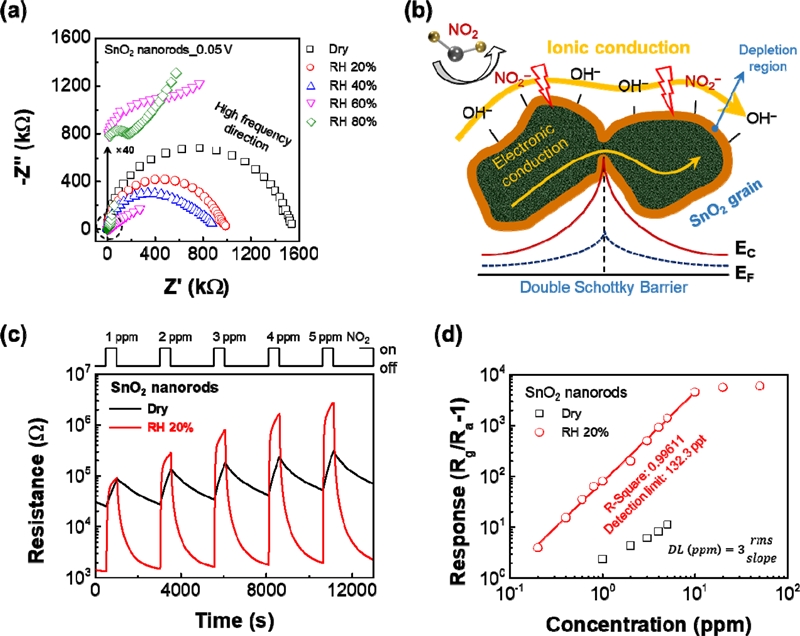
(a) Impedance spectra of the SnO2 nanorods as a function of RH 0 to 80%. (b) Ionic-activated gas sensing mechanism. (c) Response curves for 1–5 ppm NO2 in dry and RH 20%. (d) Theoretical detection limits at RH 20% [29].
Based on the ionic conduction, the ionic-activated mechanism is operated by the modulation of ionic conductivity upon the reaction of NO2 gas on the surface (Fig. 4b). In addition, Fig. 4c exhibits room-temperature gas-sensing properties with different NO2 concentrations under a dry condition and a relative humidity of 20%. Because of the ionic-activated mechanism, the sensor shows extremely ideal gas-sensing properties, including a high response (1402 for 5-ppm NO2) and fast recovery (25.7 s). With increasing the relative humidity, the response were steadily decreased (259, 60.2, and 13.9 at RH 40, 60, 80%). At the high relative humidity conditions, the sensor surface was covered with H2O physisorbed multipayer. Thus, NO2 must penetrate the multilayer to interact with the SnO2 surface, resulting in degradation of the gas response. According to Song et al., the humidity undoubtedly accelerates desorption of the pre-adsorbed, which is replaced by hydroxide ions because (Ea,NO2−Ea,OH) is smaller than (Ea,NO2−Ea,O2). Therefore, the hydroxide layer is an effective receptor for improve the recovery at room temperature. In addition, the theoretical detection limit was found to be 132.3 ppt with the response linearity, which was confirmed by an R-squared of 0.99438, as shown in Fig. 4d. This showed an entirely different mechanism compared with the conventional sensors, and it pioneered a new field of ionic-activated sensors.
4. CONCLUSIONS
Important progress has been achieved recently for room-temperature semiconducting gas sensors using nanostructured materials, 2D materials, UV-activated methods, and so on. In this review, the room-temperature gas sensors were briefly discussed, and state-of-the-art ionic-activated gas sensors were introduced with theoretical background. Through these various strategies, it should be possible to make progress toward the development of ideal gas sensors for room-temperature operation.
Acknowledgments
This work was supported by an Institute for Information & Communications Technology Promotion (IITP) grant funded by the Korean government (MSIP; Ministry of Science, ICT & Future Planning; No. 2019-0-00725, Development of Non-contact Dementia Screening and Cognitive Enhancer Content Technology), and the National Research Foundation of Korea(NRF) grant funded by the Korea Government(MSIT) (No. 2019R1A2B5B01070286).
References
-
L. Atzori, A. Iera, and G. Morabito, “The internet of things: A survey”, Comput. Netw., Vol. 54, No. 15, pp. 2787-2805, 2010.
[https://doi.org/10.1016/j.comnet.2010.05.010]

-
R. A. Potyrailo, “Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet”, Chem. Rev., Vol. 116, No. 19, pp. 11877-11923, 2016.
[https://doi.org/10.1021/acs.chemrev.6b00187]

-
X. Liu, S. Cheng, H. Liu, S. Hu, D. Zhang, and H. Ning, “A survey on gas sensing technology”, Sensors, Vol. 12, No. 7, pp. 9635-9665, 2012.
[https://doi.org/10.3390/s120709635]

-
S. Lakkis, R. Younes, Y. Alayli, and M. Sawan, “Review of recent trends in gas sensing technologies and their miniaturization potential”, Sens. Rev., Vol. 34, No. 1, pp. 24-35, 2014.
[https://doi.org/10.1108/SR-11-2012-724]

-
N. Minh Triet, L. Thai Duy, B.-U. Hwang, A. Hanif, S. Siddiqui, K.-H. Park, C.-Y. Cho, and N.-E. Lee, “High-Performance Schottky Diode Gas Sensor Based on the Heterojunction of Three-Dimensional Nanohybrids of Reduced Graphene Oxide–Vertical ZnO Nanorods on an AlGaN/GaN Layer”, ACS Appl. Mater. Interfaces, Vol. 9, No. 36, pp. 30722-30732, 2017.
[https://doi.org/10.1021/acsami.7b06461]

-
H. Nazemi, A. Joseph, J. Park, and A. Emadi, “Advanced micro-and nano-gas sensor technology: A review”, Sensors, Vol. 19, No. 6, pp. 1285, 2019.
[https://doi.org/10.3390/s19061285]

- S. Capone, A. Forleo, L. Francioso, R. Rella, P. Siciliano, J. Spadavecchia, D. Presicce, and A. Taurino, “Solid state gas sensors: state of the art and future activities”, J. Optoelectron. Adv. Mater., Vol. 5, No. 5, pp. 1335-1348, 2003.
-
C. Wang, L. Yin, L. Zhang, D. Xiang, and R. Gao, “Metal oxide gas sensors: sensitivity and influencing factors”, Sensors, Vol. 10, No. 3, pp. 2088-2106, 2010.
[https://doi.org/10.3390/s100302088]

-
S. J. Patil, A. V. Patil, C. G. Dighavkar, K. S. Thakare, R. Y. Borase, S. J. Nandre, N. G. Deshpande, and R. R. Ahire, “Semiconductor metal oxide compounds based gas sensors: A literature review”, Front. Mater. Sci., Vol. 9, No. 1, pp. 14-37, 2015.
[https://doi.org/10.1007/s11706-015-0279-7]

-
K. Lee, Y.-S. Shim, Y. Song, S. Han, Y.-S. Lee, and C.-Y. Kang, “Highly sensitive sensors based on metal-oxide nanocolumns for fire detection”, Sensors, Vol. 17, No. 2, pp. 303, 2017.
[https://doi.org/10.3390/s17020303]

-
Z. Yuan, R. Li, F. Meng, J. Zhang, K. Zuo, and E. Han, “Approaches to Enhancing Gas Sensing Properties: A Review”, Sensors, Vol. 19, No. 7, pp. 1495, 2019.
[https://doi.org/10.3390/s19071495]

-
T. Rault, A. Bouabdallah, and Y. Challal, “Energy efficiency in wireless sensor networks: A top-down survey”, Comput. Netw., Vol. 67, No.4, pp. 104-122, 2014.
[https://doi.org/10.1016/j.comnet.2014.03.027]

-
J. Zhang, X. Liu, G. Neri, and N. Pinna, “Nanostructured materials for room-temperature gas sensors”, Adv. Mater., Vol. 28, No. 5, pp. 795-831, 2016.
[https://doi.org/10.1002/adma.201503825]

-
W. Choi, N. Choudhary, G. H. Han, J. Park, D. Akinwande, and Y. H. Lee, “Recent development of two-dimensional transition metal dichalcogenides and their applications”, Mater. Today, Vol. 20, No. 3, pp. 116-130, 2017.
[https://doi.org/10.1016/j.mattod.2016.10.002]

- N. Yamazoe, G. Sakai, and K. Shimanoe, “Oxide semiconductor gas sensors”, Catal. Surv. Asia, Vol. 7, No. 1, pp. 63-75, 2003.
-
M. Batzill, “Surface science studies of gas sensing materials: SnO2”, Sensors, Vol. 6, No. 10, pp. 1345-1366, 2006.
[https://doi.org/10.3390/s6101345]

- N. Barsan, and U. Weimar, “Conduction model of metal oxide gas sensors”, J. Electroceram., Vol. 7, No. 3, pp. 143-167, 2001.
-
Y. G. Song, J. Y. Park, J. M. Suh, Y.-S. Shim, S. Y. Yi, H. W. Jang, S. Kim, J. M. Yuk, B.-K. Ju, and C.-Y. Kang, “Heterojunction Based on Rh-Decorated WO3 Nanorods for Morphological Change and Gas Sensor Application Using the Transition Effect”, Chem. Mater., Vol. 31, No. 1, pp. 207-215, 2018.
[https://doi.org/10.1021/acs.chemmater.8b04181]

-
S. Y. Yi, Y. G. Song, J. Y. Park, J. M. Suh, G. S. Kim, Y.-S. Shim, J. M. Yuk, S. Kim, H. W. Jang, and B.-K. Ju, “Morphological Evolution Induced through a Heterojunction of W-Decorated NiO Nanoigloos: Synergistic Effect on High-Performance Gas Sensors”, ACS Appl. Mater. Interfaces, Vol. 11, No. 7, pp. 7529-7538, 2019.
[https://doi.org/10.1021/acsami.8b18678]

-
S. Bianchi, E. Comini, M. Ferroni, G. Faglia, A. Vomiero, and G. Sberveglieri, “Indium oxide quasi-monodimensional low temperature gas sensor”, Sens. Actuators B, Vol. 118, No. 1-2, pp. 204-207, 2006.
[https://doi.org/10.1016/j.snb.2006.04.023]

-
X. Pan, X. Zhao, J. Chen, A. Bermak, and Z. Fan, “A fast-response/recovery ZnO hierarchical nanostructure based gas sensor with ultra-high room-temperature output response”, Sens. Actuators B, Vol. 206, pp. 764-771, 2015.
[https://doi.org/10.1016/j.snb.2014.08.089]

-
X. Tang, A. Du, and L. Kou, “Gas sensing and capturing based on two-dimensional layered materials: Overview from theoretical perspective”, Wiley Interdiscip. Rev. Comput. Mol. Sci., Vol. 8, No. 4, pp. e1361, 2018.
[https://doi.org/10.1002/wcms.1361]

-
H. Li, Z. Yin, Q. He, H. Li, X. Huang, G. Lu, D. W. H. Fam, A. I. Y. Tok, Q. Zhang, and H. Zhang, “Fabrication of single-and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature”, Small, Vol. 8, No. 1, pp. 63-67, 2012.
[https://doi.org/10.1002/smll.201101016]

-
K. C. Kwon, J. M. Suh, T. H. Lee, K. S. Choi, K. Hong, Y. G. Song, Y.-S. Shim, M. Shokouhimehr, C.-Y. Kang, and S. Y. Kim, “SnS2 Nanograins on Porous SiO2 Nanorods Template for Highly Sensitive NO2 Sensor at Room Temperature with Excellent Recovery”, ACS Sensors, Vol. 4, No. 3, pp. 678-686, 2019.
[https://doi.org/10.1021/acssensors.8b01526]

-
E. Espid, and F. Taghipour, “UV-LED photo-activated chemical gas sensors: A review”, Crit. Rev. Solid State Mater. Sci., Vol. 42, No. 5, pp. 416-432, 2017.
[https://doi.org/10.1080/10408436.2016.1226161]

-
S.-W. Fan, A. K. Srivastava, and V. P. Dravid, “UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO”, Appl. Phys. Lett., Vol. 95, No. 14, pp. 142106, 2009.
[https://doi.org/10.1063/1.3243458]

-
R. Kumar, N. Goel, and M. Kumar, “UV-activated MoS2 based fast and reversible NO2 sensor at room temperature”, ACS Sensors, Vol. 2, No. 11, pp. 1744-1752, 2017.
[https://doi.org/10.1021/acssensors.7b00731]

-
S. Park, S. An, Y. Mun, and C. Lee, “UV-enhanced NO2 gas sensing properties of SnO2-core/ZnO-shell nanowires at room temperature”, ACS Appl. Mater. Interfaces, Vol. 5, No. 10, pp. 4285-4292, 2013.
[https://doi.org/10.1021/am400500a]

-
Y. G. Song, Y. S. Shim, J. M. Suh, M. S. Noh, G. S. Kim, K. S. Choi, B. Jeong, S. Kim, H. W. Jang, and B. K. Ju, “Ionic-Activated Chemiresistive Gas Sensors for Room-Temperature Operation”, Small, Vol. 15, No., pp. 1902065-1902073, 2019.
[https://doi.org/10.1002/smll.201902065]

-
X. Song, Q. Qi, T. Zhang, C. Wang, “A humidity sensor based on KCl-doped SnO2 nanofibers”, Sens. Actuators B Chem., Vol. 138, No. 1, pp. 368-373, 2009.
[https://doi.org/10.1016/j.snb.2009.02.027]
