Effect of Noble Metals on Hydrogen Sensing Properties of Metal Oxide-based Gas Sensors
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
As a green and abundant source of energy, H2 has attracted the attention of researchers for use in different applications. Nevertheless, it is highly flammable, and because of its significantly small size, extreme attention is needed to detect its leakage. In this review, we discuss different effects of noble metals on the H2 gas response and performance of metal oxide-based gas sensors. In this regard, we discuss the effects of noble metals, in combination with metal oxides, on H2 gas detection. The catalytic activity towards H2 gas and the formation of heterojunctions with metal oxides are the main contributions of noble metals to the sensing improvement of H2 gas sensors. Furthermore, in the special case of Pd and somewhat Pt, the formation of PdHx and PtHx also affects the H2 sensing performance. This review paper provides useful information for researchers working in the field of H2 gas detection.
Keywords:
Noble metal, Metal oxide, Gas sensor1. Hydrogen Gas and hydrogen Gas sensors
H2 constitutes ~75% of the mass of the universe, and upon combustion, it only produces H2O [1]. Thus, it is regarded as a clean, abundant, and renewable energy source [2]. It is used in hydrogen batteries [3], fuel cells [4], etc. [5]. However, it is a highly explosive gas due to low ignition temperature (520-580°C) and energy (~0.017 mJ), large flame propagation energy, high heat of combustion (286 kJ/mol), and wide air flammable range (4– 74%) [6,7]. Moreover, at high concentrations, hydrogen will hinder an adequate supply of oxygen, causing asphyxiation [8]. Owing to such features, along with no color, odor, and taste [9], rapid and accurate detection of hydrogen is of importance in various fields. In this context, different hydrogen gas sensors such as electrochemical [10], catalytic combustion [11], thermoelectric [12], optical [13], gasochromic [14, 15], colorimetric [16], Pd-film and Pd-alloy films [17], and metal oxide-based [18], have been developed. Among them, metal oxide gas sensors are highly popular because of their high sensitivity, high stability, fast response time, and low price [19,20]. However, the main disadvantage of metal oxide sensors is their poor selectivity. In this regard, different approaches, such as p-n junction formation [21] and noble metal decoration [22], have been used to enhance the selectivity toward a specific gas. Herein, we only focus on the decoration of noble metals to enhance the hydrogen gas sensing properties of metal oxides.
2. Noble Metal Decoration
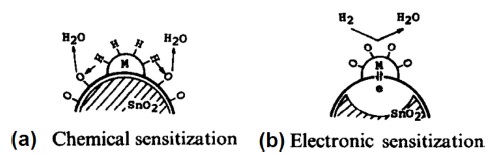
Noble metals can be decorated on the surface of host sensing materials using various methods such as UV-reduction [23], gamma-ray irradiation [24], chemical reduction [25], and sputtering [26]. Regardless of the decoration method, generally two mechanisms, namely, chemical sensitization and electronic sensitization, are responsible for the enhancement of hydrogen gas sensing properties in the presence of noble metals [27,28]. Chemical sensitization mainly manifests as a catalytic effect [29]. In this type of sensitization, as shown in Fig. 1 (a), hydrogen molecules dissociate into hydrogen atoms on the surface of noble metal NPs, and then spill over (migrate) to the surface of the host material [30], thus accelerating the gas sensing reactions through subsequent reaction with the already adsorbed oxygen ions at the surface of the gas sensor [31,32]:
| (1) |
Hydrogen atoms are transported to the metal oxide through diffusion and can access additional sites on the surface of the metal oxide sensing layer. Obviously, the creation of more intimate contacts between the noble metal NPs and the metal oxides can enhance the response of the gas sensor [33]. Zhu et al. reported that without Pt NPs, self-heated W18O49 gas sensors did not show any response to H2 gas, demonstrating the promising catalytic role of Pt for H2 gas [34]. Noble metals such as Pt and Pd are particularly active for oxidation reactions because the heat of adsorption of oxygen on noble metals is sufficiently low to allow relatively low activation energy of oxidation and consequently a rapid rate of reactions. Thus, it is expected that the optimal sensing temperature of noble metal-decorated hydrogen gas sensors is lower than that of pristine gas sensors [35].
Electronic sensitization occurs through a direct electronic interaction between the noble metal NPs and the metal oxide surface (Fig. 1 (b)). When the oxidation state of the noble metal changes with the surrounding atmosphere, the electronic state of the metal oxide changes accordingly. More specifically, typical sensitizers of this type (Ag and Pd) are known to form stable oxides (AgxO and PdOx) in air, while they are easily reduced to metals with a reducing gas. The observed work-function shifts indicate that each promoter in the oxidized form produces a strongly electron-depleted layer inside the metal oxide, while the electronic interaction is inhibited when it is reduced to metal. However, since Pt and Au generally cannot form stable oxides under these conditions, they do not show such sensitization [28].
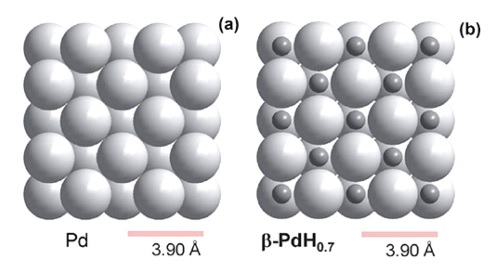
As a hydrogen reactant, Pd has a particular affinity for hydrogen. Pd can uptake more than 600 times its own volume of hydrogen gas [36]. When hydrogen appears near Pd NPs, molecular H2 dissociates into atomic H, which is characterized by a high dissociation rate. At low concentrations of H2 gas, a solid solution of H2 in the host metal (α-phase) is formed. With further increase of H2, a new phase called β-phase starts forming, where both the α-phase and β-phase co-exist in equilibrium. A further increase in the H2 concentration will result in the growth of the β- phase region at the expense of the α-phase. Finally, when the concentration of hydrogen reaches a certain value, the entire Pd is transformed into the β-phase (PdHx), as shown in Fig. 2.
The overall reaction can be represented as follows [38, 39]:
| (2) |
Lee et al. [40] reported the hydrogen-sensing properties of Pddecorated In2O3-ZnO nanofibers. As shown in Fig. 3, initially in air, Pd-ZnO Schottky barriers were formed; subsequently, in the presence of H2 gas, the formation of PdHx with a very different work function (3.7 eV) than Pd destroyed the Schottky barriers to form Ohmic contacts, which eventually resulted in a highly modulated sensor resistance.
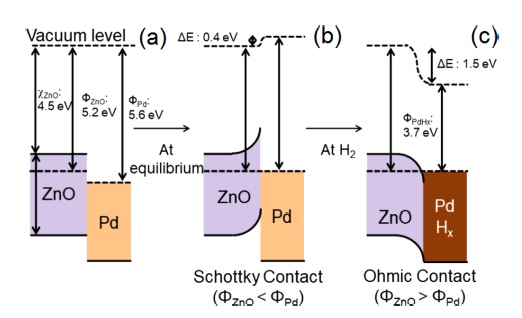
(a) Energy levels of Pd and ZnO before contact and after contact in (a) air and (b) hydrogen atmosphere [40].
It should be noted that the formation of PdHx leads to changes in the physical properties of metallic Pd, including changes in volume and electrical resistivity. Therefore, pristine Pd thin films were also used for the detection of hydrogen gas [41,42]. Similarly, Pt can form PtHx in the presence of hydrogen gas [43]. However, the degree of hydrogen adsorption is much lower than that of Pd.
3. Conclusions
Noble metal decoration is a promising approach to enhance the hydrogen gas sensing properties of metal oxide gas sensors. Noble metal NPs well-dispersed on the surface of metal oxides can affect the response towards hydrogen gas primarily through chemical sensitization and electronic sensitization. Furthermore, Pd and Pt noble metals can adsorb hydrogen atoms, leading to significant changes in the work function upon exposure to hydrogen gas and subsequent electronic interactions with the host metal oxide, leading to an improvement in the response to hydrogen gas.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03013422), and supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2019R1A2C1006193).
References
-
M. Momirlan and T. N. Veziroglu, “The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet”, Int. J. Hydrog. Energy, Vol. 30, No. 7, pp. 795-802, 2005.
[https://doi.org/10.1016/j.ijhydene.2004.10.011]

-
F. Dawood, M. Anda, and G. Shafiullah, “Hydrogen production for energy: An overview”, Int. J. Hydrog. Energy, Vol. 45, No. 7, pp. 3847-3869, 2020.
[https://doi.org/10.1016/j.ijhydene.2019.12.059]

-
Z. Zhu, M. Wang, Y. Meng, Z. Lin, Y. Cui, and W. Chen, “A High-Rate Lithium Manganese Oxide-Hydrogen Battery”, Nano Lett., Vol. 20, No. 5, pp. 3278-3283, 2020.
[https://doi.org/10.1021/acs.nanolett.0c00044]

-
P. Ahmadi, S. H. Torabi, H. Afsaneh, Y. Sadegheih, H. Ganjehsarabi, and M. Ashjaee, “The effects of driving patterns and PEM fuel cell degradation on the lifecycle assessment of hydrogen fuel cell vehicles”, Int. J. Hydrog. Energy, Vol. 45, No. 5, pp. 3595-3608, 2020.
[https://doi.org/10.1016/j.ijhydene.2019.01.165]

-
A. M. Abdalla, S. Hossain, O. B. Nisfindy, A. T. Azad, M.Dawood, and A. K. Azad, “Hydrogen production, storage,transportation and key challenges with applications: A review”, Energy Conv. Manag., Vol. 165, pp. 602-627, 2018.
[https://doi.org/10.1016/j.enconman.2018.03.088]

-
W. J. Buttner, M. B. Post, R. Burgess, and C. Rivkin, “An overview of hydrogen safety sensors and requirements”, Int. J. Hydrog. Energy, Vol. 36, No. 3, pp. 2462-2470, 2011.
[https://doi.org/10.1016/j.ijhydene.2010.04.176]

-
A. Umar, H. Ammar, R. Kumar, T. Almas, A. A. Ibrahim, M. AlAssiri, M. Abaker, and S. Baskoutas, “Efficient H2 gas sensor based on 2D SnO2 disks: experimental and theoretical studies”, Int. J. Hydrog. Energy, Vol. 45, No. 50, pp. 26388-26401, 2020.
[https://doi.org/10.1016/j.ijhydene.2019.04.269]

-
K. Mazloomi and C. Gomes, “Hydrogen as an energy carrier: prospects and challenges”, Renew. Sustain. Energy Rev., Vol. 16, No. 5, pp. 3024-3033, 2012.
[https://doi.org/10.1016/j.rser.2012.02.028]

-
B. Jang, W. Kim, M.-J. Song, and W. Lee, “Thermal stability of the sensing properties in H2 sensors composed of Pd nanogaps on an elastomeric substrate”, Sens. Actuator B, Vol. 240, pp. 186-192, 2017.
[https://doi.org/10.1016/j.snb.2016.08.140]

-
G. Korotcenkov, S. D. Han, and J. R. Stetter, “Review of electrochemical hydrogen sensors”, Chem. Rev., Vol. 109, No. 3, pp. 1402-1433, 2009.
[https://doi.org/10.1021/cr800339k]

-
A. S. Pranti, D. Loof, S. Kunz, V. Zielasek, M. Bäumer, and W. Lang, “Characterization of a highly sensitive and selective hydrogen gas sensor employing Pt nanoparticle network catalysts based on different bifunctional ligands”, Sens. Actuator B, Vol. 322, pp. 128619(1)-128619(11), 2020.
[https://doi.org/10.1016/j.snb.2020.128619]

-
M. Matsumiya, F. Qiu, W. Shin, N. Izu, N. Murayama, and S. Kanzaki, “Thin-film Li-doped NiO for thermoelectric hydrogen gas sensor”, Thin Solid Films, Vol. 419, No. 1-2, pp. 213-217, 2002.
[https://doi.org/10.1016/S0040-6090(02)00762-9]

-
S. S. Kalanur, Y.-A. Lee, and H. Seo, “Eye-readable gasochromic and optical hydrogen gas sensor based on CuS– Pd”, RSC Adv., Vol. 5, No. 12, pp. 9028-9034, 2015.
[https://doi.org/10.1039/C4RA11067F]

-
N. Matsuyama, S. Okazaki, H. Nakagawa, H. Sone, and K. Fukuda, “Response kinetics of a fiber-optic gas sensor using Pt/WO3 thin film to hydrogen”, Thin Solid Films, Vol. 517, No. 16, pp. 4650-4653, 2009.
[https://doi.org/10.1016/j.tsf.2009.01.126]

-
F. T. Foroushani, H. Tavanai, M. Ranjbar, and H. Bahrami, “Fabrication of tungsten oxide nanofibers via electrospinning for gasochromic hydrogen detection”, Sens. Actuator B, Vol. 268, pp. 319-327, 2018.
[https://doi.org/10.1016/j.snb.2018.04.120]

-
Y. K. Kim, S.-H. Hwang, S. M. Jeong, K. Y. Son, and S. K. Lim, “Colorimetric hydrogen gas sensor based on PdO/ metal oxides hybrid nanoparticles”,Talanta, Vol. 188, pp. 356-364, 2018.
[https://doi.org/10.1016/j.talanta.2018.06.010]

-
K. Hassan, A. I. Uddin, and G. -S. Chung, “Fast-response hydrogen sensors based on discrete Pt/Pd bimetallic ultrathin films”, Sens. Actuator B, Vol. 234, pp. 435-445, 2016.
[https://doi.org/10.1016/j.snb.2016.05.013]

-
U. T. Nakate, R. Ahmad, P. Patil, Y. Yu, and Y.-B. Hahn, “Ultra thin NiO nanosheets for high performance hydrogen gas sensor device”, Appl. Surf. Sci., Vol. 506, pp. 144971, 2020.
[https://doi.org/10.1016/j.apsusc.2019.144971]

-
A. Dey, “Semiconductor metal oxide gas sensors: A review”, Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater., Vol. 229, pp. 206-217, 2018.
[https://doi.org/10.1016/j.mseb.2017.12.036]

-
P. T. Moseley, “Progress in the development of semiconducting metal oxide gas sensors: a review”, Sci. Technol., Vol. 28, No. 8, pp. 082001(1)- 082001(15), 2017.
[https://doi.org/10.1088/1361-6501/aa7443]

-
X.-T. Yin, J. Li, D. Dastan, W.-D. Zhou, H. Garmestani, and F. M. Alamgir, “Ultra-high selectivity of H2 over CO with a pn nanojunction based gas sensors and its mechanism”, Sens. Actuator B, Vol. 319, pp. 128330(1)- 128330(9), 2020.
[https://doi.org/10.1016/j.snb.2020.128330]

-
Y. K. Moon, S.-Y. Jeong, Y. C. Kang, and J.-H. Lee, “Metal oxide gas sensors with Au nanocluster catalytic overlayer: toward tuning gas selectivity and response using a novel bilayer sensor design”, ACS Appl. Mater. Interfaces, Vol. 11, No. 35, pp. 32169-32177, 2019.
[https://doi.org/10.1021/acsami.9b11079]

-
J.-H. Lee, J.-Y. Kim, J.-H. Kim, A. Mirzaei, H. W. Kim, and S. S. Kim, “Pd-decorated Si nano-horns as sensitive and selective hydrogen gas sensors”, Mater. Res. Bull., Vol. 132, pp. 110985(1)- 110985(7), 2020.
[https://doi.org/10.1016/j.materresbull.2020.110985]

-
J.-H. Kim, A. Mirzaei, H. W. Kim, and S. S. Kim, “Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires”, Sens. Actuator B, Vol. 267, pp. 597-607, 2018.
[https://doi.org/10.1016/j.snb.2018.04.079]

-
A. Kaniyoor, R. I. Jafri, T. Arockiadoss, and S. Ramaprabhu, “Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor”, Nanoscale, Vol. 1, No. 3, pp. 382-386, 2009.
[https://doi.org/10.1039/b9nr00015a]

-
M. Matsumiya, W. Shin, N. Izu, and N. Murayama, “Nanostructured thin-film Pt catalyst for thermoelectric hydrogen gas sensor”, Sens. Actuator B,Vol. 93, No. 1, pp. 309-315, 2003.
[https://doi.org/10.1016/S0925-4005(03)00223-5]

-
A. Esfandiar, S. Ghasemi, A. Irajizad, O. Akhavan, and M. Gholami, “The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing”, Int. J. Hydrog. Energy, Vol. 37, No. 20, pp. 15423-15432, 2012.
[https://doi.org/10.1016/j.ijhydene.2012.08.011]

-
N. Yamazoe, “New approaches for improving semiconductor gas sensors”, Sens. Actuator B, Vol. 5, No. 1, pp. 7- 19, 1991.
[https://doi.org/10.1016/0925-4005(91)80213-4]

-
S. Dhall, K. Sood, and R. Nathawat, “Room temperature hydrogen gas sensors of functionalized carbon nanotubes based hybrid nanostructure: role of Pt sputtered nanoparticles”, Int. J. Hydrog. Energy, Vol. 42, No. 12, pp. 8392- 8398, 2017.
[https://doi.org/10.1016/j.ijhydene.2017.02.005]

-
R. Prins, “Hydrogen spillover. Facts and fiction”, Chem. Rev., Vol. 112, No. 5, pp. 2714-2738, 2012.
[https://doi.org/10.1021/cr200346z]

-
Z. Li, Z. Yao, A. A. Haidry, T. Plecenik, L. Xie, L. Sun, and Q. Fatima, “Resistive-type hydrogen gas sensor based on TiO2: A review”, Int. J. Hydrog. Energy, Vol. 43, No. 45, pp. 21114-21132, 2018.
[https://doi.org/10.1016/j.ijhydene.2018.09.051]

-
M. S. Barbosa, P. H. Suman, J. J. Kim, H. L. Tuller, J. A. Varela, and M. O. Orlandi, “Gas sensor properties of Ag- and Pd-decorated SnO micro-disks to NO2, H2 and CO: catalyst enhanced sensor response and selectivity”, Sens. Actuator B,Vol. 239, No. 45, pp. 253-261, 2017.
[https://doi.org/10.1016/j.snb.2016.07.157]

-
L. Wang and R. T. Yang, “New sorbents for hydrogen storage by hydrogen spillover–a review”, Energy Environ. Sci., Vol. 1, No. 2, pp. 268-279, 2008.
[https://doi.org/10.1039/b807957a]

-
L. F. Zhu, J. C. She, J. Y. Luo, S. Z. Deng, J. Chen, X. W. Ji, and N. S. Xu, “Self-heated hydrogen gas sensors based on Pt-coated W18O49 nanowire networks with high sensitivity, good selectivity and low power consumption”, Sens. Actuator B, Vol. 153, No. 2, pp. 354-360, 2011.
[https://doi.org/10.1016/j.snb.2010.10.047]

-
C. -H. Han, D.-W. Hong, I.-J. Kim, J. Gwak, S.-D. Han, and K. C. Singh, “Synthesis of Pd or Pt/titanate nanotube and its application to catalytic type hydrogen gas sensor”, Sens. Actuator B, Vol. 128, No. 1, pp. 320-325, 2007.
[https://doi.org/10.1016/j.snb.2007.06.025]

-
K. Hassan, A. I. Uddin, and G.-S. Chung, “Mesh of ultrasmall Pd/Mg bimetallic nanowires as fast response wearable hydrogen sensors formed on filtration membrane”, Sens. Actuator B, Vol. 252, No. 1, pp. 1035-1044, 2017.
[https://doi.org/10.1016/j.snb.2017.06.109]

-
A. Gurlo and D. R. Clarke, “High-sensitivity hydrogen detection: Hydrogen-induced swelling of multiple cracked palladium films on compliant substrates”, Angew. Chem. Int. Ed., Vol. 50, No. 43, pp. 10130-10132, 2011.
[https://doi.org/10.1002/anie.201103845]

-
A. Mirzaei, H. R. Yousefi, F. Falsafi, M. Bonyani, J.-H. Lee, J.-H. Kim, H. W. Kim, and S. S. Kim, “An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas", Int. J. Hydrog. Energy, Vol. 44, No. 36, pp. 20552-20571, 2019.
[https://doi.org/10.1016/j.ijhydene.2019.05.180]

-
Y.-N. Zhang, H. Peng, X. Qian, Y. Zhang, G. An, and Y. Zhao, “Recent advancements in optical fiber hydrogen sensors”, Sens. Actuator B, Vol. 244, No. 36, pp. 393-416, 2017.
[https://doi.org/10.1016/j.snb.2017.01.004]

-
J.-H. Lee, J.-H. Kim, J.-Y. Kim, A. Mirzaei, H. W. Kim, and S. S. Kim, “Ppb-Level selective hydrogen gas detection of Pd-functionalized In2O3-loaded ZnO nanofiber gas sensors”, Sensors, Vol. 19, No. 19, pp. 4276(1)-4276(12), 2019.
[https://doi.org/10.3390/s19194276]

-
Q. Liu, J. Yao, Y. Wu, Y. Wang, and G. Ding, “Two operating modes of palladium film hydrogen sensor based on suspended micro hotplate”, Int. J. Hydrog. Energy, Vol. 44, No. 21, pp. 11259-11265, 2019.
[https://doi.org/10.1016/j.ijhydene.2019.02.228]

-
H.-J. Noh, H.-J. Kim, Y. M. Park, J.-S. Park, and H.-N. Lee, “Complex behavior of hydrogen sensor using nanoporous palladium film prepared by evaporation”, Appl. Surf. Sci., Vol. 480, No. 21, pp. 52-56, 2019.
[https://doi.org/10.1016/j.apsusc.2019.02.088]

-
N. X. Thai, N. Van Duy, N. Van Toan, C. M. Hung, N. Van Hieu, and N. D. Hoa, “Effective monitoring and classification of hydrogen and ammonia gases with a bilayer Pt/ SnO2 thin film sensor”, Int. J. Hydrog. Energy, Vol. 45, No. 3, pp. 2418-2428, 2020.
[https://doi.org/10.1016/j.ijhydene.2019.11.072]