
Skin-interfaced Wearable Biosensors: A Mini-Review
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Wearable devices have the potential to revolutionize future medical diagnostics and personal healthcare. The integration of biosensors into scalable form factors allow continuous and noninvasive monitoring of key biomarkers and various physiological indicators. However, conventional wearable devices have critical limitations owing to their rigid and obtrusive interfaces. Recent developments in functional biocompatible materials, micro/nanofabrication methods, multimodal sensor mechanisms, and device integration technologies have provided the foundation for novel skin-interfaced bioelectronics for advanced and user-friendly wearable devices. Nonetheless, it is a great challenge to satisfy a wide range of design parameters in fabricating an authentic skin-interfaced device while maintaining its edge over conventional devices. This review highlights recent advances in skin-compatible materials, biosensor performance, and energy-harvesting methods that shed light on the future of wearable devices for digital health and personalized medicine.
Keywords:
personal healthcare, biosensors, wearable devices, multimodal sensor, micro/nanofabrication, skin-interfaced bioelectronics, digital health1. INTRODUCTION
With the ongoing global outbreak of coronavirus, the demand for improvements in personalized healthcare and telehealth systems has emerged significantly [1-3]. Conventional healthcare systems are highly centralized in local hospitals and medical institutions; hence, issues with accessibility have surfaced during the pandemic. Patients suffering from diseases have had difficulties finding adequate care and essential treatment due to a shortage of medical staff—the COVID overload. The apparent shortcomings of current health systems worldwide have highlighted the need for systematic improvements and accelerated transitions into digital and personalized healthcare platforms.
The most efficient way to reduce health risks is through preliminary diagnosis; however, people visit their doctors only when they start suffering from aggravated symptoms. Unfortunately, pain-accompanying symptoms usually occur past the preliminary stage of a disease. For patients diagnosed with chronic conditions, such as diabetes and cardiovascular disease, it is necessary to periodically monitor their health conditions at home. However, repetitive check-ups and medical tests can be difficult and inaccurate owing to their intermittency. For hospitalized patients, frequent medical tests and devices monitoring their vitals, which are attached to their body parts, can cause pain and discomfort. These problems strongly validate the demand for nonintrusive medical devices to continuously monitor health conditions without causing distress to patients.
Skin-interfaced electronics and their applications in digital healthcare are promising solutions [4]. Through the integration of sensor arrays on a biocompatible form factor, various biophysical (pressure, strain, temperature), biochemical (glucose, pH, lactate, ions, electrolytes, metabolites), and electrophysical (electroencephalogram (ECG), electroencephalogram (EMG), electroencephalogram (EEG)) signals can be measured continuously for out-of-hospital care. By significantly enhancing the comfort and versatility of rigid conventional wearable medical devices, future-generation skin-interfaced electronics can enhance patients’ experiences both in and out of the hospital.
A reliable self-powered device integrated into a biocompatible interface that can transmit real-time data is the epitome of a skin-interfaced bioelectronic device. However, multiple challenges exist in the development of an ideal device owing to its stringent dimensional and material constraints and unstable working conditions. Investigations across several fields report feasible designs at the laboratory level; however, the reliability of the device must be validated, and the fabrication cost of the system must be economical for its practical use as a medical device. Nonetheless, ongoing research indicates encouraging prospects in the field of wearable biosensors and their applications in future digital healthcare systems. Herein, a comprehensive overview of the recent advances in functional biocompatible materials, multimodal wearable biosensors, and efficient energy-harvesting mechanisms has been presented.
2. FUNCTIONAL BIOCOMPATIBLE MATERIALS
The fundamental backbone of skin-interfaced electronics is their material composition. The appropriate integration of substrates, sensing elements, and nanostructures can enhance functional features and, most importantly, dictate the usability of the entire system. Popular materials incorporated into flexible devices are based on combinations of polymers, hydrogels, liquid metals/ionic liquids, and functional nanomaterials
2.1 Polymers
Substrates for flexible devices are usually fabricated using stretchable polymeric matrices, such as polydimethylsiloxane, poly(vinylpyrrolidone), Ecoflex, polyethylene terephthalate, and polyimide (PI) [4,11]. These materials are commonly used as dielectric layers in capacitive sensors. Moreover, intrinsically conductive polymers, including poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) (PEDOT: PSS) [12,25], diketopyrrolopyrrole [13], and polyaniline [14], are used as active layers in various biosensors.
2.2 Hydrogels
Hydrogels are composed of a three-dimensional network composed of a hydrophilic polymer that provides a device interface with enhanced biocompatibility [6]. It offers effective mechanical, electrical, optical, thermal, and chemical coupling between biological tissues and the device [15]. The electrical properties of functional hydrogel interfaces can be modified by mixing conductive additives. Another unique feature that upholds hydrogel applications is its self-healing capability. However, the multifaceted advantages of hydrogels have trade-offs that must be compensated for. For instance, physical crosslinking can enable good self-healing properties but may result in low stiffness. Nonetheless, applications that require direct contact with biological tissues, such as chronic wound monitoring devices may benefit from the biocompatibility of functional hydrogels [34].
2.3 Liquid metals/Ionic liquids
Liquid metals and ionic liquids are highly conductive materials with controllable fluid properties. Inherent material compatibility issues between substrates and sensing materials in solid-state devices can be solved by integrating liquid-state conductors such as Galinstan (GaInSn) [16,17] and ethylene glycol/sodium chloride [18]. The integration of these liquid materials with precise nanoprinting techniques has enabled the fabrication of physical sensors based on conductive liquids.
2.4 Functional nanomaterials
Owing to their superior physical and chemical properties and versatile features, functional nanomaterials have been widely applied to enhance the sensor performance in various ways. Advancements in the synthesis techniques for distinctive morphologies (nanoparticles, nanowires, and thin films) have made this integration feasible. Various micro/nanomaterials such as carbon nanoparticles, silver nanoparticles, carbon nanotubes (CNT), silver nanowires, gold nanowires, and graphene nanosheets have been used to enhance sensor performance factors, such as the sensitivity, response time, and linearity of sensor signals [9].
3. WEARABLE BIOSENSORS
Wearable biosensors react to external stimuli, generated due to fluctuations in physiological conditions and observe possible biomarkers. The skin is the largest organ in the body, and various biomarkers such as uric acid, pH, glucose, ion concentration, and temperature can be obtained noninvasively. Hence, biosensors integrated into a skin interface can detect numerous types of key biological signals. This section discusses three main types of biosensors, as summarized in Table 1 (physical [16-30], chemical [31-35], and optical [36-41]).
3.1 Physical biosensors
Physical sensors attached on the skin surface focus on changes in physical stimuli such as pressure, strain, and temperature. Through this information, arterial pulse rate, blood pressure, body temperature, human motion, and tactile feedback can be analyzed. [4,5,8,9]
A key function of skin-interface electronics is the measurement of pressure and strain. Stretchable physical sensors have achieved significant performance quality with various sensing mechanisms, such as piezoresistive [20,21], capacitive [16, 22-23], piezoelectric [43, 46], and triboelectric measurements [44-46]. Among these types of sensors, piezoresistive and capacitive methods are favored because of the simplicity of their design and versatility in acquiring both static and dynamic data [6].
In piezoresistive pressure sensors, the resistance of the conductive active layer depends on its geometry, formation, and composition. The applied strain can be measured using an active transducer by measuring the resistance variation under loading. [8,9] Piezoresistive devices have multifunctional applications across a wide range of pressures and strains with good sensor characteristics, including high linearity, sensitivity, reliability, and fast response time. The integration of nanomaterials (graphene, carbon black, CNT, nanowires, nanoparticles, ionic liquids, and liquid metals) into the active layer and micro-engineered geometrical designs (porous, microcrack, auxetic) [4,5,8,9] have enhanced the piezoresistive sensor performance. Fig. 1a illustrates a CNT-coated porous elastomer-based flexible pressure sensor with an ultrawide pressure sensing range (10 Pa–1.2 MPa), high sensitivity (0.01 – 0.02 kPa-1), and good linearity (R2~0.98) [20]. Next, Fig. 1b portrays a battery-free wireless pressure and temperature sensing platform that incorporates a near-field communication system-on-a-chip to monitor immobilized patients for their risk of developing ulcers [21].
Capacitive sensors measure the change in capacitance between two electrodes, as physical deformation affects the geometries and dielectric layer properties of the sensor. This simplistic mechanism offers high sensitivity and reliability, while requiring low power to measure strain and pressure. To improve sensor performance, micropatterned structures (pores, pyramids, domes, ridges, papillae, spheres, and cylinders) were implemented on the dielectric elastomer layer [4,5,24]. These designs allow enhanced sensitivity and response time, while reducing viscoelastic deformation and hysteresis effects. More importantly, through dielectric layer design parameterization, sensor features can be customized according to the necessary applications. The micro-pyramidal capacitive sensor shown in Fig. 1c demonstrates a parameterized design for tunable capacitive sensors [24]. In Fig. 1d, another application of the porous Ecoflex-CNT composite is shown comprising a capacitive-type sensor with an enhanced pressure sensitivity due to the synergy of the porous structure and percolation network of the CNTs [22]. However, one critical drawback of using flexible elastomer-based dielectric materials is the cumulative increase in the elastic modulus during compression. This phenomenon drastically reduces the sensitivity and accuracy of pressure measurements in highly compressed regions.
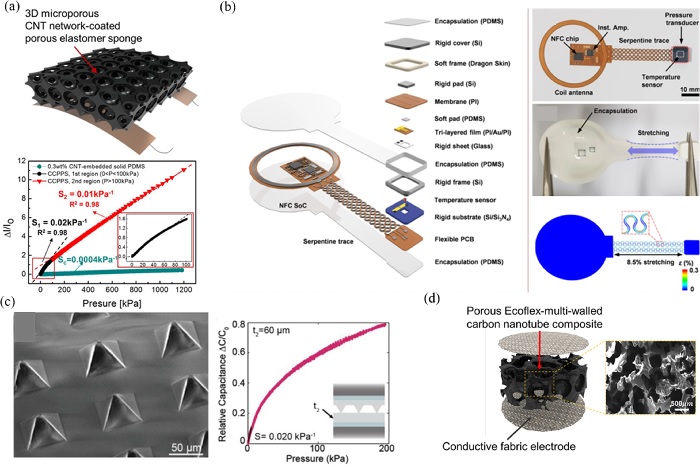
Piezoresistive and capacitive physical sensors (a) Microporous carbon nanotubes (CNT) network-coated elastomer sponge based piezoresistive pressure sensor Ref. [20] Copyright (2019) American Chemical Society. (b) Battery-free wireless pressure and temperature sensing platform to monitor patients at risk for ulcers Ref. [21] Copyright (2021) Nature Research. (c) Micro-pyramidal capacitive sensor with tunable parameter Ref. [24] Copyright (2020) John Wiley and Sons. (d) Porous Ecoflex-CNT composite based capacitive pressure sensor Ref. [22] Copyright (2020) American Chemical Society.
Body temperature is a crucial indicator of the degree of inflammation or blood flow, which determines the overall health of an individual [25-27]. Due to the ongoing pandemic, accurate body temperature monitoring has become essential. It has become common practice in worksites, restaurants, and public spaces. In fact, abnormal body temperature (high or low) is a clear indication of an unusual process occurring in the body. Common temperature-sensing devices employ infrared thermography, colorimetry, or use thermistors and thermocouples [6]. Recent studies show applications of temperature-sensitive materials such as PEDOT: PSS [25], gold [26], and CNTs [27] embedded in a flexible substrate. The change in the resistance of each material can be modeled with the temperature variation to accurately calibrate the temperature measurements. However, these active materials can also respond to mechanical strain, which is an adverse issue associated with skin-interfaced biosensors and needs to be addressed. Efforts to decouple and eliminate temperature and strain interactions in skin-attached electronics have shown progress by incorporating additional components, structural variations [21], and mathematical calibration methods [25].
Biopotential signals, such as EEG, ECG, and EMG, provide insight into brain function, heart activity, and muscle condition; however, procedural complications limit their frequent use. Recent research suggests the plausible integration of these biopotential measurements into point-of-care situations [28-30]. A highly flexible wearable cardiac sensor was designed to monitor cardiac signals wirelessly in remote and ambulatory settings [29]. This device demonstrated effective performances validated by clinical feasibility tests and intimate skin coupling by incorporating biocompatible adhesives at the skin-device interface. Moreover, simultaneous monitoring of electrophysiological signals with various biophysical/chemical data enables the fusion of different modalities for a more comprehensive assessment of health status. For instance, Wang et al. devised a hybrid sensing system (Chem-Phys patch) that simultaneously measured sweat lactate and ECG signals for real-time fitness monitoring on a single epidermal patch [28]. The promising performance of the Chem-Phys patch further demonstrated the potential of advanced hybrid multimodal sensors as wearable medical devices.
3.2 Chemical biosensors
Biomarker monitoring through the analysis of sweat, saliva, tears, interstitial fluids, and other body fluid samples provides another valuable dimension for wearable biosensors [5,7,10,11]. By applying various electrochemical analysis methods (ion-selective potentiometry, voltammetry, amperometry, bioaffinity sensing, etc.), noninvasive bioanalytical practices can be implemented [31]. The design of microfluidic structures and multisite sensor elements must be fully integrated to maintain control over the biofluid absorption and excretion. Hence, chemical biosensors require precise fabrication to ensure sensor stability. Sweat is a highly favored secreted fluid for electrochemical analysis because it is relatively hygienic, contains numerous analytes, and has easy acquisition methods [32, 33]. Fig. 2a illustrates Javey et al. ’s work [32] that incorporated hydrophilic fillers and microchannels within an enclosed wearable patch for the rapid uptake of sweat and prevention of leakage via evaporation. The microchannels are intertwined in a spiral shape and attached to electrodes that act as impedance-based sensors to measure pH, Cl-, and levodopa [32]. They also suggested a sweat and urine sample-based nutrition tracker by measuring the amount of Vitamin C and comparing it with that in blood samples [33].
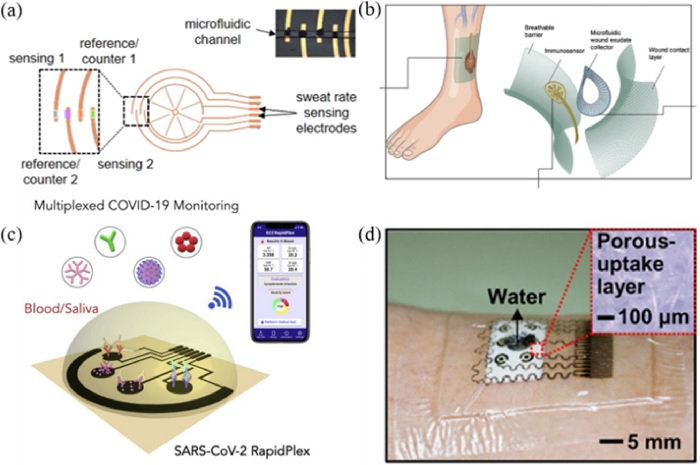
Chemical biosensors (a) Microchannels within an enclosed wearable patch for sweat uptake Ref. [32] Copyright (2021) Nature Research. (b) Soft bioaffinity sensor array for chronic wound monitoring Ref. [34] Copyright (2021) Cell Press. (c) Telemedicine platform for COVID-19 diagnosis Ref. [2] Copyright (2020) American Chemical Society. (d) Patch-based glucose monitoring wearable device Ref. [35] Copyright (2017) American Association for the Advancement of Science.
Fig. 2b shows a soft bioaffinity sensor array for chronic wound monitoring developed by Gao et al [34]. According to their study, diabetic patients face chronic problems with open wounds and the detection of inflammatory and physiological biomarkers can help treat and manage wounds [34]. Another publication suggested modes for telemedicine platforms for COVID-19 diagnosis and monitoring, as shown in Fig. 2c [2,3]. Their multisite laser-engraved graphene platform provides a portable, wireless electrochemical sensor for the rapid detection of COVID-19 in blood and saliva samples. As shown in Fig. 2d, a patch-based glucose monitoring wearable device with microneedles has been introduced for patients with diabetes. This device not only measures glucose levels but also provides feedback for drug delivery mechanisms based on the patient’s condition [35].
These studies validated the positive impacts of developing at-home personal health monitoring devices; however, critical reliability issues exist that hinder their commercial use. For instance, microfluidic channel control is susceptible to clogging problems owing to the deposition of particles dissolved in biofluids and external disruptions. Moreover, issues related to functional reliability, calibration, electrochemical selectivity, and system optimization require breakthrough solutions. Finally, the physiological data extracted from these sensors must be validated by medical experts to draw accurate conclusions.
3.3 Optoelectronic biosensors
Optoelectronic sensors use diverse optical properties to measure both biophysical and biochemical entities using photodetectors [37]. Optical sensing mechanisms mainly detect variations in phototransmittance and backscattering to process these signals into physiological information. Conventional wearable devices incorporate photoplethysmography (PPG)-based pulse oximetry to monitor the heart rate and blood oxygenation of the user, as demonstrated in Fig. 3a. [38].
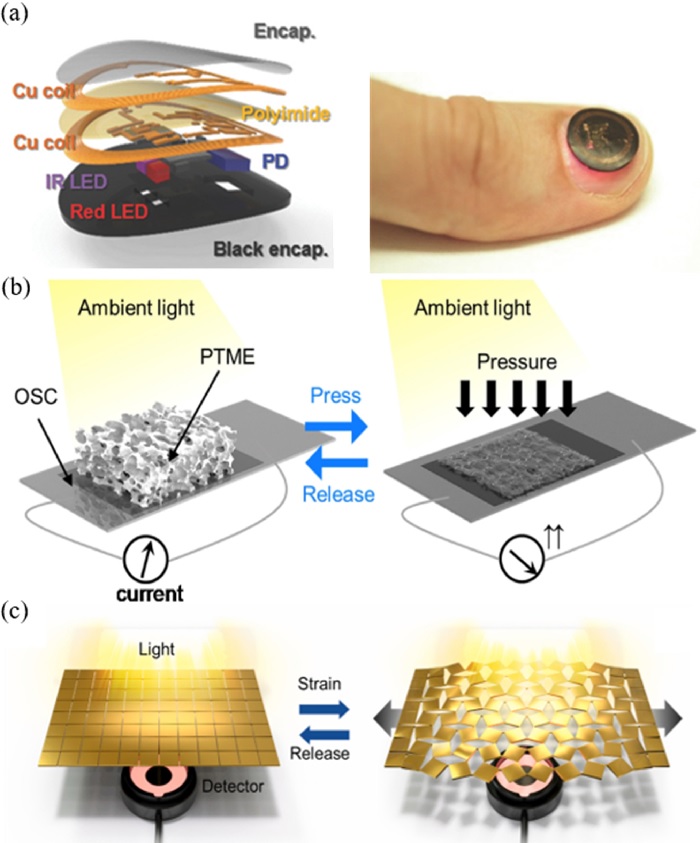
Optoelectronic biosensors (a) PPG based pulse oximetry device to monitor heart rate and blood oxygenation Ref. [38] Copyright (2017) John Wiley and Sons. (b) Piezo-transmittance based self-powered pressure sensor Ref. [40] Copyright (2020) Elsevier. (c) Auxetic metamaterial piezo-transmittance strain sensor Ref. [41] Copyright (2021) Elsevier.
Optical sensors can also be used to measure mechanical strain and pressure by using a material structure that is designed to alter optical transmittance with applied force. For example, a CNT-embedded elastomer with microcracks could detect subtle human motions using optical transmittance measurements [39]. Fig. 3b shows the application of a wearable self-powered pressure sensor that integrates a piezo-transmittance microporous elastomer with an organic solar cell [40]. The initial porous structure causes refraction of light rays, preventing the solar cell from absorbing ambient light. However, the application of pressure compresses the air gaps thereby allowing the light transmittance to increase the current output. The applied pressure was detected by monitoring the current output from the solar cell. Finally, Fig. 3c illustrates a novel implementation of auxetic metamaterials in a piezo-transmittance-based strain sensor [41]. The auxetic structure amplified the gap opening caused by strain, enhancing the sensor’s performance up to a gauge factor of 10.5.
The development of inorganic μLEDs [36], quantum dots, metamaterials, and various nanostructures has enhanced the potential of optoelectronic sensors and their feasible applications because of their fast response, sensitivity, and simplicity. However, it is important to consider the effects of undesired light sources in optoelectronic devices.
4. ENERGY HARVESTING
Self-powering is another crucial feature of future wearable electronics [10]. To continuously retrieve data from the sensing components, a stable power source is essential. However, it is ineffective for integrating a rigid battery into a skin-interfaced device. Hence, attempts have been made to fabricate a flexible battery that can be implemented in device circuitry [42,43]. Moreover, energy-harvesting methods and self-powered sensor designs are also popular subjects of investigation [44]. Energy harvesting mechanisms rely on ambient and external sources, such as thermal, solar, mechanical, and chemical energy, to power the device. Consequently, their stability heavily depends on the working conditions of the particular device of interest. To solve this issue, self-powered sensors use physical stimuli to be monitored as a source of energy harvesting.
For instance, triboelectric nanogenerators and piezoelectric nanogenerators use mechanical stimuli and convert them into electrical signals by employing piezoelectric materials (zinc oxide, lead zirconate titanate, polyvinylidene fluoride) and triboelectric materials (polymers, graphene, and CNT) into different structures and assemblies [44-47]. Similarly, optoelectronic devices incorporate self-powered photodetectors using high-performance inorganic perovskites and organic solar cells as their light intensity sensing components [40,41,48].
By incorporating various micro/nanofabrication technologies and functional materials, self-powered mechanisms can exhibit high efficiency and notable output performance. Future research aims to further optimize the energy conversion efficiency, improve various performance metrics, and enhance the direct integration modes to flexible device systems.
5. CONCLUSIONS
This review highlights recent studies related to advanced materials, novel sensor fabrication and design, and energy harvesting methods that act as a foundation for future skin-interfaced wearable devices. Finding effective ways to integrate the aforementioned technologies is still a critical task to enhance the feasibility of flexible electronics. It must be noted that these advancements do not merely suggest their potential in their distinctive fields of research but also shed light on wearable healthcare devices that are essential in adopting digital healthcare systems and revolutionary clinical procedures in the future.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C3008742).
REFERENCES
-
D. V. Gunasekeran, R. M. W. W. Tseng, and Y. C. Tham, “Applications of digital health for public health responses to COVID-19: a systematic scoping review of artificial intelligence, telehealth and related technologies,” npj Digit. Med., Vol. 4, No. 1, 2021.
[https://doi.org/10.1038/s41746-021-00412-9]

-
H. Lukas, C. Xu, and W. Gao, “Emerging telemedicine tools for remote covid-19 diagnosis, monitoring, and management,” ACS Nano, Vol. 14, No. 12, pp. 16180–16193, 2020.
[https://doi.org/10.1021/acsnano.0c08494]

-
R. M. Torrente-Rodríguez, H. Lukas, and W. Gao, “SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring,” Matter, Vol. 3, No. 6, pp. 1981–1998, 2020.
[https://doi.org/10.1016/j.matt.2020.09.027]

-
H. R. Lim, H. S. Kim, and W. H. Yeo, “Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment,” Adv. Mater., Vol. 32, No. 15, pp. 1–43, 2020.
[https://doi.org/10.1002/adma.201901924]

-
J. C. Yang, Z. Bao, and S. Park, “Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics,” Adv. Mater., Vol. 31, No. 48, pp. 1–50, 2019.
[https://doi.org/10.1002/adma.201904765]

-
C. Xu, Y. Yang, and W. Gao, “Skin-Interfaced Sensors in Digital Medicine: from Materials to Applications,” Matter, Vol. 2, No. 6, pp. 1414–1445, 2020.
[https://doi.org/10.1016/j.matt.2020.03.020]

-
T. R. Ray, J. Choi, and J. A. Rogers, “Bio-integrated wearable systems: A comprehensive review,” Chem. Rev., Vol. 119, No. 8, pp. 5461–5533, 2019.
[https://doi.org/10.1021/acs.chemrev.8b00573]

-
M. Amjadi, K. U. Kyung, and I. Park, “Stretchable, Skin-Mountable, and Wearable Strain Sensors and Their Potential Applications: A Review,” Adv. Funct. Mater., Vol. 26, No. 11, pp. 1678–1698, 2016.
[https://doi.org/10.1002/adfm.201504755]

-
H. Souri, M. Amjadi, and I. Park, “Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications,” Adv. Intell. Syst., Vol. 2, No. 8, p. 2000039, 2020.
[https://doi.org/10.1002/aisy.202000039]

-
D. Mukasa, H. Zhang, and W. Gao, “Self-Powered Wearable Biosensors,” Accounts Mater. Res., Vol. 2, No. 3, pp. 184–197, 2021.
[https://doi.org/10.1021/accountsmr.1c00002]

-
Y. Lin, M. Bariya, and A. Javey, “Wearable Biosensors for Body Computing,” Adv. Funct. Mater., Vol. 31, No. 39, pp. 1–21, 2021.
[https://doi.org/10.1002/adfm.202008087]

-
Y. Wang, C. Zhu, and Z. Bao, “A highly stretchable, transparent, and conductive polymer,” Sci. Adv., Vol. 3, No. 3, pp. 1–11, 2017.
[https://doi.org/10.1126/sciadv.1602076]

-
J. Y. Oh, J. B. Tok, and Z. Bao, “Intrinsically stretchable and healable semiconducting polymer for organic transistors,” Nature, Vol. 539, No. 7629, pp. 411–415, 2016.
[https://doi.org/10.1038/nature20102]

-
M. Jose, R. Thoelen, and W. Deferme, “Printed pH Sensors for Textile-Based Wearables: A Conceptual and Experimental Study on Materials, Deposition Technology, and Sensing Principles,” Adv. Eng. Mater., Vol. 2101087, pp. 1–15, 2021.
[https://doi.org/10.1002/adem.202101087]

-
Q. Yang, Z. Hu, and J. A. Rogers, “Functional Hydrogel Interface Materials for Advanced Bioelectronic Devices,” Accounts Mater. Res., Vol. 2, No. 11, pp. 1010–1023, 2021.
[https://doi.org/10.1021/accountsmr.1c00142]

-
K. Kim, Y. S. Oh, and I. Park, “Highly Sensitive and Wearable Liquid Metal-Based Pressure Sensor for Health Monitoring Applications: Integration of a 3D-Printed Microbump Array with the Microchannel,” Adv. Healthc. Mater., Vol. 8, No. 22, pp. 1–10, 2019.
[https://doi.org/10.1002/adhm.201900978]

-
K. Kim, J. Ahn, and I. Park, “All-soft multiaxial force sensor based on liquid metal for electronic skin,” Micro Nano Syst. Lett., Vol. 9, No. 1, 2021.
[https://doi.org/10.1186/s40486-020-00126-9]

-
O. Gul, K. Kim, and I. Park, “Sensitivity-Controllable Liquid-Metal-Based Pressure Sensor for Wearable Applications,” ACS Appl. Electron. Mater., Vol. 3, No. 9, pp. 4027–4036, 2021.
[https://doi.org/10.1021/acsaelm.1c00546]

-
D. Y. Choi, H. W. Lee, and H. M. Lee, “Highly stretchable, hysteresis-free ionic liquid-based strain sensor for precise human motion monitoring,” ACS Appl. Mater. Interfaces, Vol. 9, No. 2, pp. 1770–1780, 2017.
[https://doi.org/10.1021/acsami.6b12415]

-
S. Kim, Y. S. Oh, and I. Park, “Wearable, Ultrawide-Range, and Bending-Insensitive Pressure Sensor Based on Carbon Nanotube Network-Coated Porous Elastomer Sponges for Human Interface and Healthcare Devices,” ACS Appl. Mater. Interfaces, Vol. 11, No. 26, pp. 23639–23648, 2019.
[https://doi.org/10.1021/acsami.9b07636]

- Y. S. Oh, I. Park, and J. A. Rogers, “Battery-free, wireless soft sensors for continuous multi-site measurements of pressure and temperature from patients at risk for pressure injuries,” Nat. Commun., Vol. 12, No. 1, pp. 1–16, 2021.
-
J. Choi, Y. S. Oh, and I. Park, “Synergetic Effect of Porous Elastomer and Percolation of Carbon Nanotube Filler toward High Performance Capacitive Pressure Sensors,” ACS Appl. Mater. Interfaces, Vol. 12, No. 1, pp. 1698–1706, 2020.
[https://doi.org/10.1021/acsami.9b20097]

-
S. R. A. Ruth, L. Beker, and Z. Bao, “Rational Design of Capacitive Pressure Sensors Based on Pyramidal Microstructures for Specialized Monitoring of Biosignals,” Adv. Funct. Mater., Vol. 30, No. 29, pp. 1–12, 2020.
[https://doi.org/10.1002/adfm.201903100]

-
S. R. A. Ruth, V. R. Feig, and Z. Bao, “Microengineering Pressure Sensor Active Layers for Improved Performance,” Adv. Funct. Mater., Vol. 30, No. 39, pp. 1–31, 2020.
[https://doi.org/10.1002/adfm.202003491]

-
P. Escobedo, M. Bhattacharjee, and R. Dahiya, “Smart Bandage with Wireless Strain and Temperature Sensors and Batteryless NFC Tag,” IEEE Internet Things J., Vol. 8, No. 6, pp. 5093–5100, 2021.
[https://doi.org/10.1109/JIOT.2020.3048282]

-
R. C. Webb, A. P. Bonifas, and J. A. Rogers, “Ultrathin conformal devices for precise and continuous thermal characterization of human skin,” Nat. Mater., Vol. 12, No. 10, pp. 938–944, 2013.
[https://doi.org/10.1038/nmat3755]

-
Q. Li, L. N. Zhang, and X. Ding, “Review of Flexible Temperature Sensing Networks for Wearable Physiological Monitoring,” Adv. Healthc. Mater., Vol. 6, No. 12, pp. 1–23, 2017.
[https://doi.org/10.1002/adhm.201601371]

-
S. Imani, J. Wang, and P. P. Mercier, “A wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring,” Nat. Commun., Vol. 7, pp. 1–7, 2016.
[https://doi.org/10.1038/ncomms11650]

-
S. P. Lee, J. A. Rogers, and R. Ghaffari, “Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring,” npj Digit. Med., Vol. 1, No. 1, 2018.
[https://doi.org/10.1038/s41746-017-0009-x]

-
A. J. Casson, “Wearable EEG and beyond,” Biomed. Eng. Lett., Vol. 9, No. 1, pp. 53–71, 2019.
[https://doi.org/10.1007/s13534-018-00093-6]

-
Y. Yu, W. Gao, and A. Javey, “Flexible Electrochemical Bioelectronics: The Rise of In Situ Bioanalysis,” Adv. Mater., Vol. 32, No. 15, pp. 1–25, 2020.
[https://doi.org/10.1002/adma.201902083]

-
H. Y. Y. Nyein, N. Davis, and A. Javey, “A wearable patch for continuous analysis of thermoregulatory sweat at rest,” Nat. Commun., Vol. 12, No. 1, pp. 1–13, 2021.
[https://doi.org/10.1038/s41467-021-22109-z]

-
J. Zhao, H. Y. Y. Nyein, and A. Javey, “A Wearable Nutrition Tracker,” Adv. Mater., Vol. 33, No. 1, pp. 1–8, 2021.
[https://doi.org/10.1002/adma.202006444]

-
E. S. Sani, C. Wang, and W. Gao, “A soft bioaffinity sensor array for chronic wound monitoring,” Matter, Vol. 4, No. 8, pp. 2613–2615, 2021.
[https://doi.org/10.1016/j.matt.2021.06.018]

-
H. Lee, C. Song, and D. H. Kim, “Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module,” Sci. Adv., Vol. 3, No. 3, pp. 1–9, 2017.
[https://doi.org/10.1126/sciadv.1601314]

-
H. Zhang and J. A. Rogers, “Recent Advances in Flexible Inorganic Light Emitting Diodes: From Materials Design to Integrated Optoelectronic Platforms,” Adv. Opt. Mater., Vol. 7, No. 2, pp. 1–27, 2019.
[https://doi.org/10.1002/adom.201800936]

-
S. Cai, X. Xu, and X. Fang, “Materials and Designs for Wearable Photodetectors,” Adv. Mater., Vol. 31, No. 18, pp. 1–15, 2019.
[https://doi.org/10.1002/adma.201808138]

-
J. Kim, P. Gutruf, and J. A. Rogers, “Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry,” Adv. Funct. Mater., Vol. 27, No. 1, pp. 1–8, 2017.
[https://doi.org/10.1002/adfm.201770007]

-
J. Gu, D. Kwon, and I. Park, “Wearable Strain Sensors Using Light Transmittance Change of Carbon Nanotube-Embedded Elastomers with Microcracks,” ACS Appl. Mater. Interfaces, Vol. 12, No. 9, pp. 10908–10917, 2020.
[https://doi.org/10.1021/acsami.9b18069]

-
J. Choi, D. Kwon, and I. Park, “Wearable self-powered pressure sensor by integration of piezo-transmittance microporous elastomer with organic solar cell,” Nano Energy, Vol. 74, 104749, 2020.
[https://doi.org/10.1016/j.nanoen.2020.104749]

-
J. Gu, J. Ahn, and I. Park, “Self-powered strain sensor based on the piezo-transmittance of a mechanical metamaterial,” Nano Energy, Vol. 89, 106447, 2021.
[https://doi.org/10.1016/j.nanoen.2021.106447]

-
L. Guo, K. Wan, and G. Wei, “Recent advance in the fabrication of carbon nanofiber-based composite materials for wearable devices,” Nanotechnology, Vol. 32, No. 44, 2021.
[https://doi.org/10.1088/1361-6528/ac18d5]

-
Z. Chen, Y. Cui, and Z. Bao, “A three-dimensionally interconnected carbon nanotube-conducting polymer hydrogel network for high-performance flexible battery electrodes,” Adv. Energy Mater., Vol. 4, No. 12, pp. 1–10, 2014.
[https://doi.org/10.1002/aenm.201400207]

-
F. R. Fan, W. Tang, and Z. L. Wang, “Flexible Nanogenerators for Energy Harvesting and Self-Powered Electronics,” Adv. Mater., Vol. 28, No. 22, pp. 4283–4305, 2016.
[https://doi.org/10.1002/adma.201504299]

-
W. He, X. Fu, and R. Ma, “Recent progress of flexible/wearable self-charging power units based on triboelectric nanogenerators,” Nano Energy, Vol. 84, 105880, 2021.
[https://doi.org/10.1016/j.nanoen.2021.105880]

-
W. Xu, L. B. Huang, and J. Hao, “Environmentally Friendly Hydrogel-Based Triboelectric Nanogenerators for Versatile Energy Harvesting and Self-Powered Sensors,” Adv. Energy Mater., Vol. 7, No. 1, pp. 1–8, 2017.
[https://doi.org/10.1002/aenm.201770004]

-
X. Cao, Y. Xiong, and Z. L. Wang, “Piezoelectric Nanogenerators Derived Self-Powered Sensors for Multifunctional Applications and Artificial Intelligence,” Adv. Funct. Mater., Vol. 31, No. 33, pp. 1–31, 2021.
[https://doi.org/10.1002/adfm.202102983]

-
K. Shen, H. Xu, and J. Wu, “Flexible and Self-Powered Photodetector Arrays Based on All-Inorganic CsPbBr3 Quantum Dots,” Adv. Mater., Vol. 32, No. 22, 2020.
[https://doi.org/10.1002/adma.202000004]

