
Triboelectric Nanogenerators for Self-powered Sensors
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Self-powered sensors play an important role in everyday life, and they cover a wide range of topics. These sensors are meant to measure the amount of relevant motion and transform the biomechanical activities into electrical signals using triboelectric nanogenerators (TENGs) since they are sensitive to external stimuli such as pressure, temperature, wetness, and motion. The present advancement of TENGs-based self-powered wearable, implantable, and patchable sensors for healthcare monitoring, human body motion, and medication delivery systems was carefully emphasized in this study. The use of TENG technology to generate electrical energy in real-time using self-powered sensors has been the topic of considerable research among various leading scholars. TENGs have been used in a variety of applications, including biomedical and healthcare physical sensors, wearable devices, biomedical, human-machine interface, chemical and environmental monitoring, smart traffic, smart cities, robotics, and fiber and fabric sensors, among others, as efficient mechanical–to–electric energy conversion technologies. In this evaluation, the progress accomplished by TENG in several areas is extensively reviewed. There will be a discussion on the future of self-powered sensors.
Keywords:
Self-powered sensors, Triboelectrification, Implantable and wearable smart textiles, Healthcare monitoring1. INTRODUCTION
The term “self-powered sensor” refers to a sensor that generates energy when physically engaged without the need for an external power source. It is common knowledge that the majority of today's sensors are passive, meaning they don't give out any signals if they don't have electricity. Second, the sensor's operating power source is self-generated [1,2]. Triboelectric nanogenerator (TENG) is among the breakthrough wearable technologies that have attracted the attention of many researchers due to its considerable impact of self, high output, lightweight, design application, cheap cost, stability and durability, and so on. This is accomplished by taking into account a sensor's active and sleeping phases [3]. A sensor does not need to give out a signal every second in many scenarios, such as environmental monitoring; a signal at a particular interval would suffice. In such cases, the energy collected during the sensor's “sleep” phase might be utilized to power it once it is switched on [4]. This is a long-term operating plan. When it comes to the fast-paced lifestyles of today's working-class individuals, biomedical wearables are particularly relevant.
When travelling between tasks, a substantial amount of the day is spent, and health and fitness are often neglected [5]. Furthermore, the time-consuming procedure of seeing a doctor for a diagnosis, prescription, and treatment causes many individuals to delay visiting a doctor or getting to a clinic until it is absolutely required. As a result, there is a lot of research being done on techniques to monitor a patient's health and communicate real-time data about their health indicators to their physician [6]. In fact, the use of these wearables allows people to take a proactive approach to medicine by monitoring their health own biosignals longitudinally. This is also known as telehealth, and it has grown in popularity as a result of the Internet of Things [5].
Telemedicine is the combination of communication technologies with healthcare, in which technology is utilized to offer healthcare services [7]. Two key tendencies in medical care's future development are “Smart Medicine” and “Precision Medicine” [8]. Drugs with precise release and slow-release control may improve therapeutic targeting while also increasing patient compliance and lowering drug adverse effects. Another key research direction and objective in the treatment of cancers in the clinic is this. The self-powered drug delivery system (DDS) is a closed-loop device that can actively manage the medication's release quantity and target based on the needs of the human body. Researchers have been working on a range of self-powered DDSs in recent years since renewable energy and self-powered systems have advanced rapidly.
Herein the progress accomplished by TENG in several areas is reviewed with a discussion on the future of self-powered sensors. This review summarizes various types of TENG-based self-powered sensors for wearable and biomedical applications during their significant development in recent years with different functions and structures.
2. TENG for Self-Powered Sensors
The grating-structured TENG fabric is shown in Fig. 1(a-b), along with its integration with fiber-shaped dye-sensitized solar cells to create a complete textile-based energy harvesting system [9]. Fig. 1(b) shows large-scale all-textile pressure sensors for monitoring human motion and physiological signals [10]. Significant human body movements are thus needed to trigger device deformation/contact-separation for the production of triboelectricity, as shown in Fig. 1(b). Textile TENGs have shown exceptional superiority in mechanical energy harvesting owing to their ease of fabrication and promising output performance and self-powered sensing because of their ease of manufacture and promising output performance [11]. Fig. 1(d) shows a self-powered pressure sensor made of weaving that can detect pulse waves from the human finger tip and ear in real-time. The surface morphology is seen in a scanning electron microscope picture. The developed gadget outperforms in terms of recording and translating tiny blood pressure changes into electrical signals for the prevention and detection of cardiovascular disease [12]. Fig. 1(e) shows how self-powered and self-functional socks perform a variety of tasks, including energy collecting and storage using a hybrid integrating (PEDOT:PSS)-coated fabric TENG and lead zirconate titanate piezoelectric chip. The detection of numerous physiological signals such as gait, contact force, and perspiration level was demonstrated. Membrane-based triboelectric sensors are built with exceptionally high resolutions for surveillance and health monitoring as self-powered sensors (Fig. 1(f)). The M-TES is a compact and lightweight device that can detect air pressure changes in situations like those depicted when air pressure starts or collapses [14]. After a lengthy period of operation, the device has shown great stability, and increased sensitivity may be predicted when the gadget is further downsized. Seung et al. presented a fabric wearable TENG (W-TENG) with good output performance and mechanical resilience, as shown in Fig. 1(g). W-TENG can use human mechanical energy to drive commercial LEDs and LCD panels. Furthermore, a keyless car entry system based on W-TENG is presented that does not need any external power sources [15].
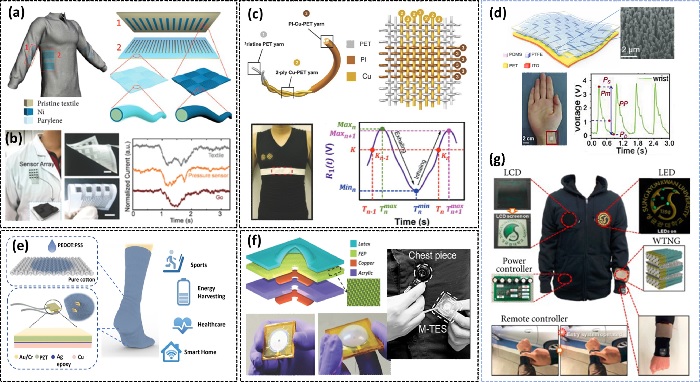
Wearable textile TENG-based physical sensors for healthcare applications. (a) The structure of a pair of fabrics TENG is depicted along with the configuration. Reprinted with permission from Ref. [9]. Copyright (2016) John Wiley and Sons. (b) Large-scale all-textile pressure sensors for human-motion monitoring. Ref. [10]. Copyright (2017) John Wiley and Sons. (c) Triboelectric yarn woven chest strap for respiratory monitoring. Reprinted with permission from Ref. [11]. Copyright (2016) John Wiley and Sons. (d) Woven constructed self-powered pressure and triboelectric sensor for subtle blood pressure monitoring. Reprinted with permission from Ref. [12]. Copyright (2019) John Wiley and Sons. (e) Smart functional socks for multi-sensing and energy harvesting. Reprinted with permission from Ref. [13]. Copyright (2019) American Chemical Society. (f) Membrane based highly stable health monitoring sensors [14]. Copyright (2014) John Wiley and Sons. (g) Nanopatterned textile-based wearable TENG for body motion sensing and charging batteries. Reprinted with permission from Ref. [15]. Copyright (2015) American Chemical Society.
Fig. 2(a-b) demonstrates the patchable TENG mechanoreceptor-inspired dynamic mechanical perception stimuli hydrogel based for dynamic sensing communicator [16,17]. The construction of a flexible, self-powered ultrasensitive pulse sensor (SUPS) based on a triboelectric active sensor with outstanding output performance, high peak signal-to-noise ratio, ultrasensitivity, and cheap cost is shown in Fig. 2(c) [18]. Without a complicated circuit design, mathematical procedures, or the consequent mistake, the SUPS can immediately acquire a voltage signal that is consistent with the second derivative of a standard pulse signal. Fig. 2(d) demonstrates the creation of active sensors inspired by the eardrum for self-powered cardiovascular and speech recognition systems that can function in several modes for health monitoring and authentication [19]. Self-powered, transparent, stretchy, ultrasensitive, and patchable strain sensors made of multifunctional nanocomposite materials for detecting diverse human activities are shown in Fig. 2(e). For stretchable power sensors, a multi-functional and solution-processable nanocomposite of low-density silver (Ag) nanowires (NWs) with a conductive elastomer was used [20]. To monitor, the skin-inspired TENG-driven self-powered multipurpose sensor is used (Fig. 2(f)). Human physiological signals, such as arterial pulse and voice vibrations. Moreover, an intelligent prosthetic hand, a self-powered pedometer/speedometer, a flexible digital keyboard, and a proof-of-concept pressure-sensor array with detecting pixels are all exhibited sequentially to further establish its broad application potential [21].
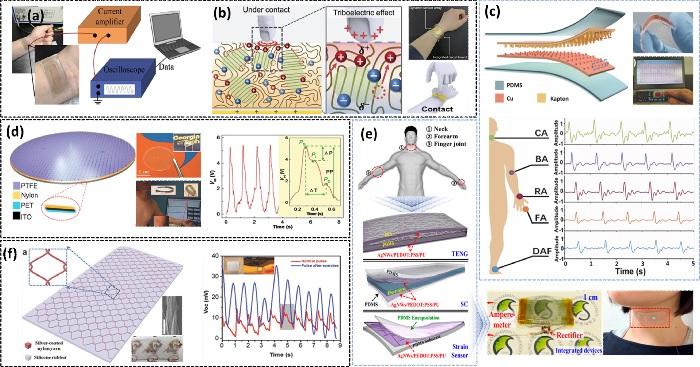
TENGs as self-powered touch/pressure sensors for applications in healthcare applications. (a) TENG-driven ultrathin, transparent, and robust electronic skins for tactile and non-contact sensing. Reprinted with permission from Ref. [16]. Copyright (2022) Elsevier. (b) An ion-doped hydrogel based patchable TENG for dynamic sensing communicator. Reprinted with permission from Ref. [17]. Copyright (2021) John Wiley and Sons. (c) Self-powered TENG-based pulse sensors with signal output of ultrasensitive pulse sensor on different artery positions. Reprinted with permission from Ref. [18]. Copyright (2018) John Wiley and Sons. (d) Eardrum-inspired active sensors for self-powered cardiovascular system characterization and voice recognition. Reprinted with permission from Ref. [19]. Copyright (2015) John Wiley and Sons. (e) Schematic representation of the stretchable TENG with Ag NWs/PEDOT:PSS/PU. Reprinted with permission from Ref. [20]. Copyright (2015) American Chemical Society. (f) A stretchable yarn embedded TENG as electronic skin (left). Real-time arterial pulse waves under normal and exercise conditions (right). Inset is the photograph of a pressure sensor attached on a wrist. Reproduced with permission from Ref. [21] Copyright (2018) John Wiley and Sons.
In recent years, self-powered biomedical devices based on TENG technology have made significant development. Fig. 3(a) shows an implanted TENG for biomechanical energy harvesting in vivo. The output voltage and matching current are driven by the heartbeat of adult pigs. A self-powered wireless transmission system was developed for real-time wireless cardiac monitoring due to its superior in vivo performance [25]. An implanted vagus nerve stimulation device that is battery-free and spontaneously sensitive to stomach movement is shown in Fig. 3(b). A flexible and biocompatible NG is mounted to the surface of the stomach in the vagus nerve stimulation system [26]. A small, flexible, ultrasensitive, and self-powered endocardial pressure sensor (SEPS) for real-time endocardial pressure monitoring is shown in Fig. 3(c). SEPS is based on a TENG, which can convert the conversion of blood flow energy into electricity inside the heart chambers The device's electric outputs, which might include EP, ventricular fibrillation, and ventricular premature contraction [27], may indicate normal and pathological cardiovascular condition. An implanted symbiotic pacemaker based on an implantable TENG (i-TENG) effectively performs cardiac pacing and sinus arrhythmia correction in a large animal model, as shown in Fig. 3(d). The body and i-TENG-based cardiac pacemaker develop a symbiotic relationship [28]. Sensors for wearable applications that are used in a variety of applications in this sector.
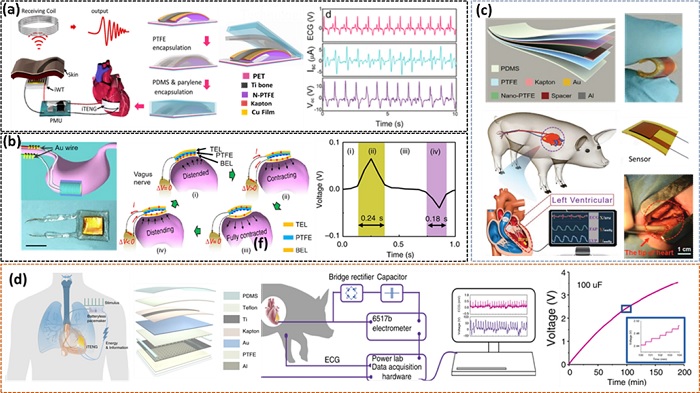
Implantable TENG devices as self-powered sensors. (a) An i-TENG for harvesting cardiac motions. Reprinted with permission from Ref. [22]. Copyright (2016) American Chemical Society. (b) Implanted vagus nerve stimulation device utilizing a TENG sensor with Au leads. Working principle of TENG based on contact mode separation under different stages of stomach peristalsis. Corresponding single cycle voltage biphasic signal produced by TENG. Reprinted with permission from Ref. [23]. Copyright (2018) Springer Nature. (c) Implantable self-powered ultrasensitive pressure sensors. Reprinted with permission from Ref. [24]. Copyright (2019) John Wiley and Sons. (d) An i-TENG for scavenging the cardiac motion through with self-powered sensing and powering a pacemaker. Reprinted with permission from Ref. [25]. Copyright (2019) Springer Nature.
3. CONCLUSIONS
The self-powered TENG-based sensors can collect biological data and serve as power sources for commercial medical sensors, enabling applications in health monitoring and physiological function regulation, such as sensors for heart rate, blood pressure, respiratory rhythm, motion, a power source for drug delivery, nerve stimulation, and more. The in vivo uses of TENGs implantable through biocompatible materials for both health monitoring and illness treatment, as well as the applications of wearable TENGs with varied structures for collecting information on physical parameters, are discussed in this study. Because of its excellent in vivo output and stability, i-TENG might be used to create a self-powered, wireless healthcare monitoring system in addition to implanted medical devices. TENG-based investigations into cell proliferation and differentiation, as well as medication delivery, are also discussed. Despite this advancement, researchers are currently attempting to determine the output performance of self-powered TENG based sensors, as well as their long-term stability, noninvasiveness, and compatibility in hard conditions for human and environmental safety. So far, the materials utilized in self-powered sensors have limits. To lower production costs and make them cheap for commercial and practical usage, fabrication techniques should be streamlined.
Acknowledgments
This work was financially supported by Nano Material Technology Development Program (2020M3H4A1A03084600) and Basic Science Research Programs (2021R1A2C2010990) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
REFERENCES
-
T. F. A. Hassani, Y. Tsuchiya, and H. Mizuta, “In-plane resonant nano-electromechanical sensors: a comprehensive study on design, fabrication and characterization challenges”, Sensors, Vol. 13, No. 7, pp. 9364-9387, 2013.
[https://doi.org/10.3390/s130709364]

-
S. Nakata, T. Arie, S. Akita, and K. Takei, “Wearable, flexible, and multifunctional healthcare device with an ISFET chemical sensor for simultaneous sweat pH and skin temperature monitoring”, ACS Sens., Vol. 2, No. 3, pp. 443-448, 2017.
[https://doi.org/10.1021/acssensors.7b00047]

-
Y. Liu, H. Wang, W. Zhao, M. Zhang, H. Qin, and Y. Xie, “Flexible, stretchable sensors for wearable health monitoring: sensing mechanisms, materials, fabrication strategies and features”, Sensors, Vol. 18, No. 2, pp. 645, 2018. B. Gil, S. Anastasova,
[https://doi.org/10.3390/s18020645]

-
B. Gil, S. Anastasova, and G.Z. Yang, and G.Z. Yang, “A smart wireless ear-worn device for cardiovascular and sweat parameter monitoring during physical exercise: design and performance results”, Sensors, Vol. 19, No.7, pp. 1616, 2019.
[https://doi.org/10.3390/s19071616]

-
F. Qureshi and S. Krishnan, “Wearable hardware design for the internet of medical things (IoMT)”, Sensors, Vol. 18, No. 11, pp. 3812, 2018.
[https://doi.org/10.3390/s18113812]

-
K. Meng, J. Chen, X. Li, Y. Wu, W. Fan, Z. Zhou, Q. He et al., “Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure”, Adv.Funct. Mater., Vol. 29, No. 5, pp. 1806388, 2019.
[https://doi.org/10.1002/adfm.201806388]

-
F. Yi, L. Lin, S. Niu, P. K. Yang, Z. Wang, J. Chen, Y. Zhou, Y. Zi, J. Wang, Q. Liao, Y. Zhang, and Z. L. Wang, “Stretchable-rubber-based TENG and its application as self-powered body motion sensors”, Adv. Funct. Mater., Vol. 25, No. 24, pp. 3688-3696, 2015.
[https://doi.org/10.1002/adfm.201500428]

-
D. Patra, S. Sengupta, W. Duan, H. Zhang, R. Pavlick, and A. Sen, “Intelligent, self-powered, drug delivery systems”, Nanoscale, Vol. 5, No. 4, pp. 1273-1283, 2013.
[https://doi.org/10.1039/C2NR32600K]

-
X. Pu, W. Song, M. Liu, C. Sun, C. Du, C. Jiang, X. Huang, D. Zou, W. Hu, and Z. L. Wang, “Wearable power-textiles by integrating fabric triboelectric nanogenerators and fiber-shaped dye-sensitized solar cells”, Adv. Energy Mater., Vol. 6, No. 20, pp. 1601048, 2016.
[https://doi.org/10.1002/aenm.201601048]

-
M. Liu, X. Pu, C. Jiang, T. Liu, X. Huang, L. Chen, C. Du, J. Sun, W. Hu, and Z. L. Wang, “Large-area all-textile pressure sensors for monitoring human motion and physiological signals”, Adv. Mater., Vol. 29, No. 41, pp. 1703700, 2017.
[https://doi.org/10.1002/adma.201703700]

-
Z. Zhao, C. Yan, Z. Liu, X. Fu, L. M. Peng, Y. Hu,and Z. Zheng, “Machine-washable textile triboelectric nanogenerators for effective human respiratory monitoring through loom weaving of metallic yarns”, Adv. Mater., Vol. 28, No. 46, pp. 10267-10274,, 2016.
[https://doi.org/10.1002/adma.201603679]

-
K. Meng, J. Chen, X. Li, Y. Wu, W. Fan, Z. Zhou, Q. He et al., “Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure”, Adv. Funct. Mater., Vol. 29, No. 5, pp. 1806388, 2019.
[https://doi.org/10.1002/adfm.201806388]

-
M. Zhu, Q. Shi, T. He, Z. Yi, Y. Ma, B. Yang, T. Chen, and C. Lee, “Self-powered and self-functional cotton sock using piezoelectric and triboelectric hybrid mechanism for healthcare and sports monitoring”, ACS Nano, Vol. 13, No.2, pp. 1940-1952, 2019.
[https://doi.org/10.1021/acsnano.8b08329]

-
P. Bai, G. Zhu, Q. Jing, J. Yang, J. Chen, Y. Su, J. Ma, G. Zhang, and Z. L. Wang, “Membrane-based self-powered triboelectric sensors for pressure change detection and its uses in security surveillance and healthcare monitoring”, Adv. Funct. Mater., Vol. 24, No. 37, pp. 5807-5813, 2014.
[https://doi.org/10.1002/adfm.201401267]

-
W. Seung, M. K. Gupta, K. Y. Lee, K. S. Shin, J. H. Lee, T. Y. Kim, S. Kim, J. Lin, J. H. Kim, and S.-W Kim, “Nanopatterned textile-based wearable TENG”, ACS Nano, Vol.9, No. 4, pp. 3501-3509, 2015.
[https://doi.org/10.1021/nn507221f]

-
R. Liu, Y. Lai, S. Li, F. Wu, J. Shao, D. Liu, X. Dong, J. Wang, and Z. L. Wang, “Ultrathin, transparent, and robust self-healing electronic skins for tactile and non-contactsensing”, Nano Energy, Vol. 95, pp. 107056, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107056]

-
H. J. Yoon, D. M. Lee, Y. J. Kim, S. Jeon, J. H. Jung, S. S. Kwak, J. Kim, S. M. Kim, Y. Kim, and S.-W. Kim, “Mechanoreceptor-inspired dynamic mechanical stimuli perception based on switchable ionic polarization”, Adv. Funct. Mater., Vol.31, No. 23, pp. 2100649, 2021.
[https://doi.org/10.1002/adfm.202100649]

-
H. Ouyang, J. Tian, G. Sun, Y. Zou, Z. Liu, H. Li, L. Zhao et al. “Self-powered pulse sensor for antidiastole of cardiovascular disease”, Adv. Mater., Vol. 29, No. 40, pp. 1703456, 2017.
[https://doi.org/10.1002/adma.201703456]

-
J. Yang, J. Chen, Y. Su, Q. Jing, Z. Li, F. Yi, X. Wen, Z. Wang, and Z. L. Wang, “Eardrum-inspired active sensors for self-powered cardiovascular system characterization and throat-attached anti-interference voice recognition”, Adv. Mater., Vol. 27, No. 8, pp. 1316-1326, 2015.
[https://doi.org/10.1002/adma.201404794]

-
B. U. Hwang, J. H. Lee, T. Q. Trung, E. Roh, D. I Kim, S.W. Kim, and N. E. Lee, “Transparent stretchable self-powered patchable sensor platform with ultrasensitive recognition of human activities”, ACS Nano, Vol. 9, No. 9, pp. 8801-8810, 2015.
[https://doi.org/10.1021/acsnano.5b01835]

-
K. Dong, Z. Wu, J. Deng, A. C. Wang, H. Zou, C. Chen, D. Hu, B. Gu, B. Sun, and Z. L. Wang, “A stretchable yarn embedded TENG as electronic skin for biomechanical energy harvesting and multifunctional pressure sensing”, Adv. Mater., Vol. 30, No. 43, pp. 1804944, 2018.
[https://doi.org/10.1002/adma.201804944]

-
Q. Zheng, H. Zhang, B. Shi, X. Xue, Z. Liu, Y. Jin, Y. Ma et al. “In vivo self-powered wireless cardiac monitoring via implantable TENG”, ACS Nano, Vol. 10, No. 7, pp. 6510-6518, 2016.
[https://doi.org/10.1021/acsnano.6b02693]

-
G. Yao, L. Kang, J. Li, Y. Long, H. Wei, C. A. Ferreira, J. J. Jeffery, Y. Lin, W. Cai, and X. Wang, “Effective weight control via an implanted self-powered vagus nerve stimulation device”, Nat. Commun., Vol. 9, No. 1, pp. 1-10, 2018.
[https://doi.org/10.1038/s41467-018-07764-z]

-
Z. Liu, Y. Ma, H. Ouyang, B. Shi, N. Li, D. Jiang, F. Xie et al. “Transcatheter self-powered ultrasensitive endocardial pressure sensor”, Adv. Funct. Mater., Vol. 29, No. 3, pp. 1807560, 2019.
[https://doi.org/10.1002/adfm.201807560]

-
H. Ouyang, Z. Liu, N. Li, B. Shi, Y. Zou, F. Xie, Ye Ma et al. “Symbiotic cardiac pacemaker”, Nat. Commun., Vol.10, No. 1, pp. 1-10, 2019.
[https://doi.org/10.1038/s41467-019-09851-1]
