Influence of Inductively Coupled Oxygen Plasma on the Surface of Poly(ether sulfone)
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The effect of inductively coupled plasma (ICP) treatment with O2 gas on the surface properties of poly(ether sulfone) (PES) was investigated. X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical characteristics of the O2 plasma-treated PES films. The surface roughness of the pristine and O2 plasma-treated PES films for different RF powers of the ICP was determined using an atomic force microscope (AFM). The contact angles of the PES films were also measured, using which the surface free energies were calculated. The O1s XPS spectra of the PES films revealed that the number of polar functional groups increased following the O2 plasma treatment. The AFM analysis showed the average surface roughness increased from 1.01 to 4.48 nm as the RF power of the ICP was increased. The contact angle measurements revealed that the PES films became more hydrophilic as the RF power of the ICP was increased. The total surface energy increased with the RF power of the ICP, resulting from the increased polar energy component.
Keywords:
Poly(ether sulfone), Surface modification, O2 plasma, Surface energy, Inductively coupled plasma1. INTRODUCTION
Recently, significant attention has been paid to flexible optoelectronic substrates due to their potential applications in wearable sensors, flexible displays, and energy harvesting [1, 2]. Polymer materials such as poly(ethylene terethalate) and poly(ethylene naphathalate) are considered good candidates for such substrates [1-4]. Poly(ether sulfone) (PES) is another good candidate for the flexible optoelectronic substrate owing to its high optical transmission in the visible wavelength range, high thermal resistance, and low manufacturing cost [5,6]. Especially, PES has great prospects in the fields of blood contact and tissue engineering [6].
Despite their excellent properties, the surfaces of the aforementioned polymers need to be modified to improve their wettability and adhesive properties for various applications [3,4,7]. To this end, oxygen (O2) plasma treatment has been commonly applied owing to its wide industrial applications. In addition, this treatment can be used for surface functionalization and etching, and the surface modification of polymers requires high oxygen density and low charged particle density. The most effective source of oxygen atoms is ionized O2 gas (or O2 plasma), which is maintained by an electrodeless discharge, such as an inductively coupled radio frequency (RF) plasma [8].
In this study, surface modification of PES films was performed using inductively coupled O2 plasma. Following O2 plasma treatment, the chemical bonds and surface roughness of the PES films were investigated. In addition, the contact angles of the O2 plasma-treated PES films were measured for different RF powers of the inductively coupled plasma (ICP). The total surface energy of the films was then obtained from the measured contact angles.
2. EXPERIMENTAL
First, PES films of thickness 200 μm were cleaned using isopropyl alcohol in an ultrasonic bath for 2min and dried using nitrogen (N2) gas. Next, the surfaces of the films were modified in an ICP reactor filled with pure O2 gas. The parameters of the ICP reactor for the surface modification of PES are summarized in Table 1.
X-ray photoelectron spectroscopy (XPS; K-Alpha, Thermo Fisher Scientific, USA) was utilized to analyze the surface chemical characteristics of the PES films. The morphology of the O2 plasma-treated PES surfaces was investigated using an atomic force microscope (AFM; XE-100, Park System, Korea). The root mean squared (RMS) surface roughness of the modified films was determined. The contact angles were measured by a drop shape analyzer (DSA; DSA 100, Krüss, Germany). The surface energy was calculated as the sum of the dispersive and polar components using the Owens Wendt geometric mean method with DI water and diiodomethane [9].
3. RESULTS AND DISCUSSIONS
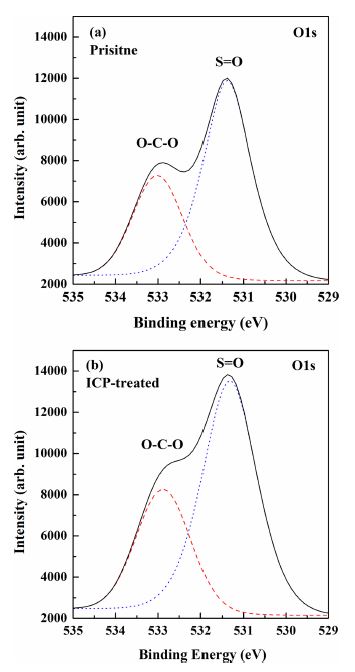
XPS analysis was utilized to study the interaction between the PES films and O2 ICP. The XPS spectra of the pristine and O2 ICP-treated PES films are shown in Fig. 1. The measured O1s peaks of both the pristine and O2 ICP-treated PES films have two prominent peaks that originated due to the O=S and O-C-O bonds. Deconvolution of the O1s spectra shows that the binding energies of the O=S and O-C-O bonds are centered at about 531.4 and 533.0 eV, respectively, for both the pristine and O2 ICP-treated PES films; however, the intensities corresponding to the O=S and O-C-O bonds are higher in the case of the O2 ICP-treated PES films. The increase in the intensities infers that oxygen ions and/or reactive radicals are incorporated into the surface of the PES during the O2 ICP treatment.
The effect of RF power on the O2 ICP-treated PES films was also investigated. Fig. 2 shows the AFM images of the surfaces of the pristine and O2 ICP-treated PES films. As shown in Fig. 2(a), the pristine PES film has a relatively smooth and uniform surface compared to the O2 ICP-treated PES films The RMS surface roughness of the pristine PES film was found to be 1.01 nm. However, O2 ICP treatment caused the surface of the PES film to become more uneven and non-uniform. At RF powers of 100, 150, and 200 W, the RMS surface roughness was measured to be 3.07, 4.21, and 4.48 nm, respectively, as shown in Figs. 2(b)-(d). Thus, the surface roughness of the O2 ICP-treated PES films increases as the RF power of the ICP increases, owing to the increased energy of the O2 ions in the plasma. Furthermore, the surface wettability of a polymer is known to be strongly correlated to surface roughness [3,4,10]. Therefore, increasing the RF power during the O2 ICP treatment would also enhance the surface wettability.
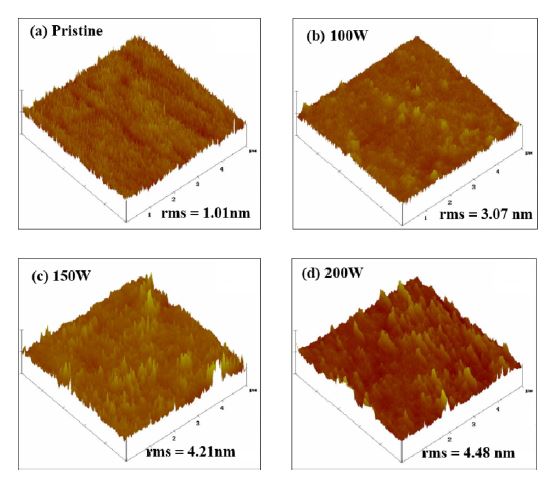
AFM images. (a) Pristine PES film. O2 ICP-treated PES films corresponding to RF powers: (b) 100 W, (c) 150 W, and (d) 200 W.
The contact angle arises owing to a thermodynamic equilibrium between the liquid, gaseous, and solid phases, and it is used to quantify the wettability of a solid surface [11,12]. Fig. 3 shows the contact angle measurements of the pristine and O2 ICP-treated PES films for different RF powers. The contact angle of the pristine PES film was found to be 86°, implying that its surface is hydrophobic. Following O2 ICP treatment, the contact angle significantly decreased and the surface of the PES films became more hydrophilic. The contact angles of the O2 ICP-treated PES films at RF powers of 100, 150, and 200 W were measured to be 34.3°, 32.5°, and 30.4°, respectively. Han et al. [3]. reported that the number of ions and reactive radicals increases with the increasing RF power of the ICP. The increase in the concentration of O2 species can decrease the contact angle.
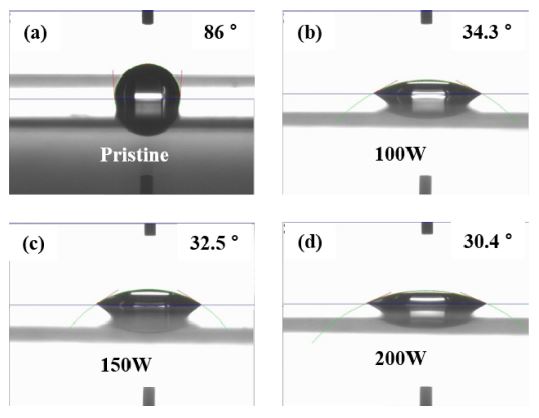
Contact angle measurements. (a) Pristine PES film. O2 ICP-treated PES films corresponding to RF powers: (b) 100 W, (c) 150 W, and (d) 200 W.
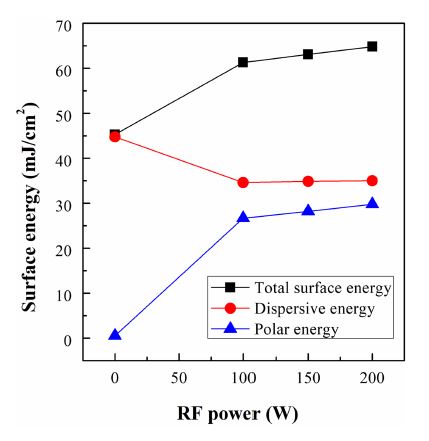
Surface properties are critical during manufacturing processes since different surfaces interact with each other surfaces or the environments for adhesion, cleaning, bonding, coating, and other processes [13]. The total surface energy and its components in the pristine and O2 ICP-treated PES films are presented in Fig. 4. Dispersive energy is the main component of the surface energy the pristine PES films. Following O2 ICP treatment, the dispersive component of the surface energy was found to decrease; however, the dispersive energy was almost unaffected by the change in RF power. The total surface energy gradually increased with increasing RF power, due to the increased polar energy. Note that the surface energy can be increased by not only increasing the number of hydrophilic functional groups on the PES surface but also by increasing the surface roughness. As shown in Fig. 3, the number of hydrophilic functional groups on the PES surface increases because of the incorporation of oxidized species on the polymer surface [14]. Also, the surface roughness of the PES films increases due to the bombardment of O2 ions in the plasma, as shown in Fig. 2.
4. CONCLUSIONS
In this study, the effect of inductively coupled O2 plasma treatment on the surface properties of PES films was investigated. The XPS analysis showed that the O1s peaks of the pristine and O2 plasma-treated PES films did not differ significantly. The AFM analysis revealed that the surface roughness of the PES films increased from 1.01 to 4.48 nm when the RF power of the O2 ICP was increased from 0 to 200 W. Moreover, O2 plasma treatment with increasing RF power made the PES films more hydrophilic. The total surface energy of the PES films was obtained from contact angle measurements, which also increased with RF power owing to the increase in the polar energy component. This study suggests that surface modification by the O2 ICP makes the PES films hydrophilic, showing the potential for flexible electronic device applications. Nevertheless, for practical applications, further investigations on the stability of the O2 ICP-treated PES films are required from the viewpoint of reliability.
Acknowledgments
This work was supported by research grants from Daegu Catholic University in 2021.
REFERENCES
-
K. E. Cheon, D. Y. Lee, Y. R. Cho, P. K. Song, and G. H. Lee, “Effect of Sputtering Conditions on the Mechanical Property and the Permeability of IZO Grown by DC Magnetron Sputtering for Application to Flexible OLEDs”, J. Korean Phys. Soc., Vol. 53, No. 1, pp. 396-401, 2008.
[https://doi.org/10.3938/jkps.53.396]

-
J. Li, L. Hu, J. Liu, L. Wang, T. J. Marks, and G. Grüner, “Indium tin oxide modified transparent nanotube thin films as effective anodes for flexible organic light-emitting diodes”, Appl. Phys. Lett.., Vol. 93, No. 8, pp. 083306(1)-083306(12), 2008.
[https://doi.org/10.1063/1.2970049]

-
D. C. Han, Y. C. Choi, H. J. Shin, S. Son, J. H. Kim, S. H. Sohn, and D. K. Lee, “Surface modification of poly(ethylene terephthalate) polymeric films by inductively coupled oxygen plasma”, Mol. Cryst. Liq. Cryst., Vol. 532, No. 1, pp. 148-155, 2010.
[https://doi.org/10.1080/15421406.2010.497061]

-
D. C. Han, Y. C. Choi, H. J. Shin, G. Kwak, K.-S. Ahn, J. H. Kim, and D. K. Lee, “Surface properties of poly(ethylene terephthalate) films modified by inductively coupled plasma with Ar/N2 mixture gases”, Mol. Cryst. Liq. Cryst., Vol. 539, No.1, pp. 210-217, 2011.
[https://doi.org/10.1080/15421406.2011.566129]

-
N. T. Vo, P. J. Yoo, G.-R. Yi, M. Schroeder, and D. Kim, “Transparent poly(ether sulfone) nanocomposite film with low thermal expansion coefficient for flexible display substrates”, Compos. B Eng., Vol. 224, pp. 109164(1)-109164(12), 2021.
[https://doi.org/10.1016/j.compositesb.2021.109164]

-
L. Wang, T. Gong, W. Ming, X. Qiao, W. Ye, L. Zhang, and C. J. Pan, “One step preparation of multifunctional poly (ether sulfone) thin films with potential for wound dressing”, Biomater. Adv., Vol. 136, pp. 212758(1)-109164(11), 2022.
[https://doi.org/10.1016/j.bioadv.2022.212758]

-
J. S. Eom and S. H. Kim, “Plasma surface treatment of polyimide for adhesive Cu/80Si20Cr/PI flexible copper clad laminate”, J. Thin Solid Films, Vol. 516, No. 14, pp. 4530-4534, 2008.
[https://doi.org/10.1016/j.tsf.2008.01.022]

-
G. Primc, D. Lojen, A. Vesel, M. Mozetič, and R. Zaplotnik, “Oxygen atom density in a large reactor powered by four inductively coupled plasma sources”, Vacuum, Vol. 199, pp. 110964(1)-110964(7), 2022.
[https://doi.org/10.1016/j.vacuum.2022.110964]

-
A. Rudawska and E. Jacniacka, “Analysis for determining surface free energy uncertainty by the Owen–Wendt method”, Int. J. Adhes. Adhes., Vol. 29, No. 4, pp. 451-457, 2009.
[https://doi.org/10.1016/j.ijadhadh.2008.09.008]

-
R. N. Wenzel, “Resistance of solid surfaces to wetting by water”, Ind. Eng. Chem., Vol. 28, No. 8, pp. 988-992, 1936.
[https://doi.org/10.1021/ie50320a024]

- https://en.wikipedia.org/wiki/Contact_angle, (retrieved on June. 17, 2022).
-
C. Y. Tong, Y. S. Chang, B. S. Ooi, and D. J. C. Chan, “Physico-chemistry and adhesion kinetics of algal biofilm on polyethersulfone (PES) membrane with different surface wettability”, J. Environ. Chem. Eng., Vol. 9, No. 6, pp. 106531(1)-106531(14), 2021.
[https://doi.org/10.1016/j.jece.2021.106531]

- https://www.brighton-science.com/blog/what-is-the-difference-between-surface-free-energy-and-surface-energy, (retrieved on June. 18, 2022).
-
I. Mrsic, T. Bäuerle, S. Ulitzsch, G. Lorenz, K. Rebner, A. Kandelbauer, and T. Chassé, “Oxygen plasma surface treatment of polymer films—Pellethane 55DE and EPR-g-VTMS”, Appl. Surf. Sci., Vol. 536, pp. 147782(1)-147782(11), 2021.
[https://doi.org/10.1016/j.apsusc.2020.147782]