
Self-powered Sensors based on Piezoelectric Nanogenerators
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Flexible, wearable, and implantable electronic sensors have started to gain popularity in improving the quality of life of sick and healthy people, shifting the future paradigm with high sensitivity. However, conventional technologies with a limited lifespan occasionally limit their continued usage, resulting in a high cost. In addition, traditional battery technologies with a short lifespan frequently limit operation, resulting in a substantial challenge to their growth. Subsequently, utilizing human biomechanical energy is extensively preferred motion for biologically integrated, self-powered, functioning devices. Ideally suited for this purpose are piezoelectric energy harvesters. To convert mechanical energy into electrical energy, devices must be mechanically flexible and stretchable to implant or attach to the highly deformable tissues of the body. A systematic analysis of piezoelectric nanogenerators (PENGs) for personalized healthcare is provided in this article. This article briefly overviews PENGs as self-powered sensor devices for energy harvesting, sensing, physiological motion, and healthcare.
Keywords:
Piezoelectric energy harvesters, Self-powered sensors, Nanogenerators, Stretchable, Implantable, Flexible healthcare monitoring1. INTRODUCTION
Piezoelectric nanogenerators (PENGs) were proposed in 2006 [1] and have rapidly progressed to considerable interest in the last century. From a materials perspective, inorganic piezoelectric materials have been widely used in manufacturing the PENG or energy harvesting or working as self-powered sensors [2]. Piezoelectric energy harvesters have attracted significant attention. New possibilities for integrating high-quality electronic systems into a single tiny device are being created by recent breakthroughs in materials, mechanics, and manufacturing techniques. The piezoelectric effect of piezoelectric materials, initially discovered by the Curie brothers in 1880, is the essential operating mechanism of PENGs [3]. Self-powered sensing is a significant application of piezoelectric materials in addition to energy harvesting. PENGs can be used directly as sensors to detect and measure external force-related stimuli, such as pressure, strain, vibration, acceleration, acoustic waves, etc. The piezoelectric equation shows a quantifiable direct relationship between the electrical voltage signal of the piezoelectric material and then strain or Strain. Energy harvesting, mainly NG technology-based self-powered systems, is essential for low-power electronics, the Internet of Things, human-machine interaction, wearable electronics, and biomedical devices. Here, PENG-based sensors got privileged for harvesting mechanical energy (or piezoelectric effect) to drive various sensors (or enhance sensing performance). Various publications on using PENGs in various specialized study fields have been published in recent years. Zhao et al. reported the piezoelectric material based flexible NGs for wearable electronic applications, which launched an investigation into novel piezoelectric components, tools, and technologies [4]. Numerous self-powered sensing applications based on PENGs have been developed owing to their improved piezoelectric performance. The PENG-based active functional sensors are grouped into four categories according to their function: artificial intelligence, biomedical applications, environmental monitoring, and human motion tracking [5].
Most sensors used in recent sensor technologies require electricity to function regularly. Realizing the self-powered operation of sensor nodes in sensor networks, wireless communication, and signal transmission is therefore crucial. In addition to serving as an excellent sustainable power source for electronic equipment, PENGs also produce an electrical signal that may be employed to sense the applied mechanical movement directly. PENGs can also operate, extend for various mechanical motions, and act as active sensors. With the development of new soft electronics, flexible sensors and systems can be incorporated into the human body or skin (by sticking-on or wearable solutions). These techniques—such as ocular motion, joint motion, and gesture monitors—have been widely utilized to track human motion. This is crucial for sports training and fitness management., etc.). Applications for human motion sensing are significant in various industries, including healthcare, human-machine interaction, and “intelligent” systems [7]. A quantified electrical signal that may be further evaluated can provide extensive information on the motion quality and kind for use in sports. PENGs can be attached to the neck, fingers, wrists, elbows, and knees, among other places, and they can help track joint motions, including the bending angle, deformation amplitude, and frequency. Smaller muscles that regulate the eye and eyelid can also be observed with PENGs, like joints. Lee et al. created an ultrathin PENG integration of aligned ZnO with nanowire compressed arrays that can serve as an active sensor for tracking ocular motion [8].
2. MATERIALS, STRUCTURAL DESIGN AND APPLICATIONS OF PENGs
Fig. 1(a) represents schematics of dissolution of low dipole and high dipole moment solvents into poly (vinylidene fluoride-trifluorethylene) P(VDF-TrFE). In this dissolving process, the improved orientation of the chains and better crystallinity were observed with a high dipole moment solution. Therefore, we proceeded with a high dipole moment solvent for further experiments. 1(b) shows the output voltage of piezoelectric devices fabricated with the high dipole moment solvent [9]. Fig. 1(c) illustrates transition metal dichalcogenides (TMDs) based energy harvesting device, as MoS2 is expected to exhibit piezoelectric properties due to its semiconducting nature and upon external stress applied to the material. The atoms of Mo and S are arranged in a non-centrosymmetric manner. For further confirmation of the piezoelectricity of MoS2, Monolayers were synthesized via chemical vapor deposition (CVD), and then PENG devices were fabricated for experimental measurements. These MoS2-based piezo devices are advantageous for powering low power consuming and self-powered sensing devices [10]. Fig. 1(d) represents an actual image of the PENG along with patching on the neck for bending movement sensing due to its flexibility and easily stacked on the body for motion observations. [11]. Fig. 1(e) illustrates the integration of CVD-grown well-aligned ZnO nanowires on a flexible PdAu-coated plastic substrate with PENG. After fabrication, the PENG device was coated with sealing material to prevent external interaction and losses. This article explains the coupling mechanism of semiconducting properties of ZnO nanowires with piezoelectricity in a sound-driven energy harvesting device [12]. Fig. 1(f) shows active sensors that are suitable for the targeted application on various human body locations. Since the displacement of the body movement is relatively significant, PENGs can easily detect it. The PENG-based autonomous human motion sensors can detect both qualitative and quantitative motion data [13]. Fig. 3 (g) shows how the sensor device was stably deformed to react to blood vessel movements because of the ultrathin plastic with high flexibility, as seen in the inset. The piezoelectric sensor is also conformally fixed to detect carotid vein pulses and another physiological activity to a person's neck pulse sensor in situ detects radial artery pulse signals before (red line), after (blue line), and the generated peak-to-peak voltage Vpp from the carotid artery pulse (top panel). At 400 and 1000 mV, Fig. 1(h), representing the bottom panel, shows how saliva is swallowed, respectively, [14].
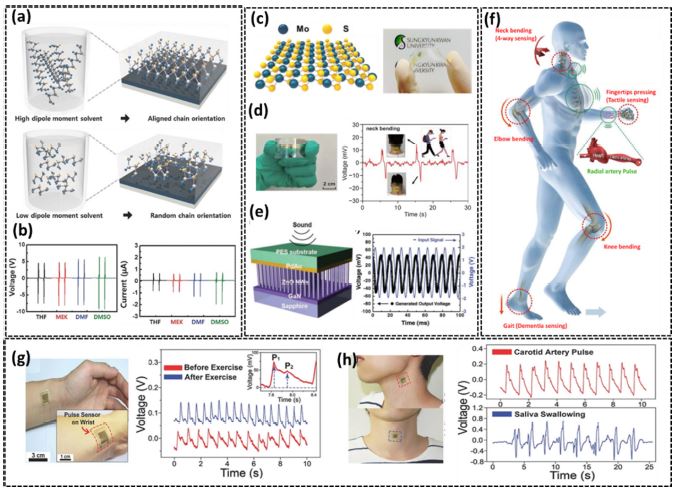
(a) An illustration of P(VDF-TrFE) liquified in a solvent with a low dipole moment (left) and the corresponding P(VDF-TrFE) film (right); an illustration of P(VDF-TrFE) dissolved in a solvent with a high dipole moment (left) and the corresponding P(VDF-TrFE) film (right); P(VDF-TrFE), (b) Voltage output measurements of PENG with high dipole moment. Reprinted with permission from Ref. [9], (c) Pictorial illustration of monolayer MoS2 atomic structure and flexible piezoelectric NG through CVD-grown monolayer MoS2 film. Reprinted with permission from Ref. [10], (d) Photograph of the flexible PENG device and Neck bending movement sensing. Reprinted with permission from Ref. [11], (e) Graphical representation of an integrated nanogenerator with ZnO nanowire arrays. Generation of an electrical output signal from ZnO nanowire array as sound waves produces the input signal. Reprinted with permission from Ref. [12], (f) Schematic illustration of wearable self-powered sensor applied to the body. Reprinted with permission from Ref. [13], (g) Conformally attached piezoelectric pulse sensors to the wrist via biocompatible liquid strapping. The inset illustrates the consistency of the deformed sensor with blood vessel movement. Radial artery pulse signals detected by the self-powered pulsation device demonstrate numerous heart rates and output voltages earlier and following physical activity. (h) The conformally attached piezoelectric pulse sensor represents a carotid blood vessel spot (top) and the center of the esophagus (throat). The carotid arterial pressure (top) and saliva swallowing actions (bottom) respond to electrical signals through sensors. Reprinted with permission from Ref. [14].
3. PENGs FOR BIOMECHANICAL MOTION SENSING
Variable aspect ratio Sb-doped ZnO nanowire films were created by Huo et al. using a low-temperature hydrothermal process. They reported their research results based on the piezoelectric effect to create a flexible, self-powered strain sensor [15]. This sensor tracks different motions and detects information about strain. Fig. 2(a) demonstrates how the output current is proportional to the degree of finger bending. To depict the signal movements, a multichannel data system was employed to measure and display the electrical signals generated by various motions demonstrated in Fig. 2(b)-(c) [16]. The entirely human-hand bendable PZT-NH2 NP-based PENG is shown in Fig. 2(d), which demonstrates its exceptional stretchability and stability during deformation. The PENG implemented device also operates as a bending-based energy harvester for renewable energy production or as a bending-motion sensor. Peak output readings were taken at the wrist and elbow of each subject using a PZT-NH2 NP-based PENG. Chou et al. demonstrated a SPENG device embedded in a standard cotton wristband, with electrical wires connecting the electrodes to the wireless communication module. SPENG exhibits different stress distributions when the wrist bends in different directions (up, down, left, and right) and generates the piezoelectric signals corresponding to wave parameters such as peak height and peak width in Fig. 2(e) [17]. A digital photograph of a piezoelectric composite nanogenerator (PCNG) device is shown in Fig. 2(f). The schematic of a self-powered sleep monitoring sensing device implanted at different points on the human body, along with the real images of the patched device shown in Fig. 2(f). The patchable devices generate electrical signals from the leg and hand motion from a person's leg and hand movements during sleep. The results confirmed that PCNG is a suitable candidate for sensing body movement, which is in progression to a smart healthcare diagnosis and treatment system [18].
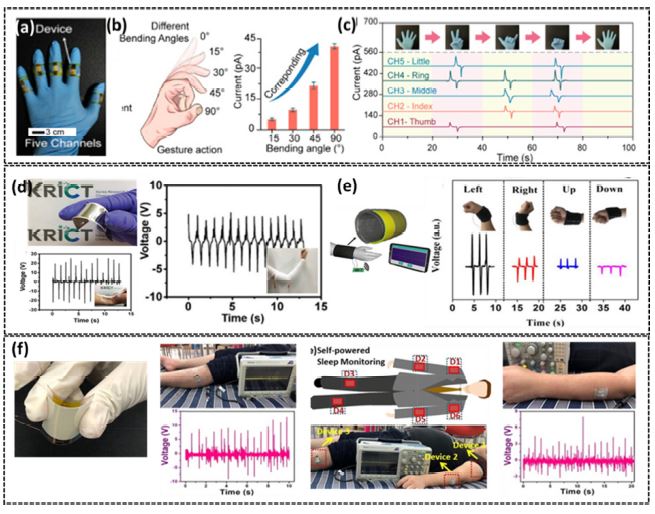
(a) Five different gadgets attached to the five fingers of a hand to build a multichannel system. (b) A schematic gesture action diagram and the current of the device shown are calculated as functions of the bending angle formed by the twisting of the index finger (97.5% confidence interval). (c) Detection of complex gesture motion by the device demonstrated by testing multiple signals with a multichannel Ref. [15]. (d) Photograph of flexible PZT-NH2 NP-based PENG device. The generated output voltage (open-circuit voltage) from the PENG device is attached to the (i) elbow and (ii) wrist when subjected to bending Ref. [16]. (e) The schematic diagram of the wireless “smart wrist band and its output signals of the “smart wrist band” under different wrist gestures Ref. [17]. (f) Photograph of the PCNG device (i) real-time sleep monitoring system with leg motion (ii) Schematic and device position of self-powered sleep monitoring and (iii) real-time sleep monitoring system with a hand motion Ref. [18].
4. PENGs AS IMPLANTABLE SELF-POWERED SENSORS
Fig. 3(a) shows a schematic of an artificial heart pacemaker employing a bendable PMN-PT thin film stimulator. To provide electrical stimulation to an anesthetized rat’s heart, a flexible cardiac stimulator was directly connected to the stimulation electrodes [19]. The in vivo test results for cardiac stimulation and heartbeat sensitivity are shown in Fig. 3(b). The ECG amplitude shown in the inset in Fig. 3(c) indicates that the rat had a typical QRS complex, P wave, and T wave. It had a heart rate of approximately six beats per second. Fig. 3(d) shows an EGC depicting the corresponding peaks in the regular heartbeat of the rat when the PMN-PT-based stimulating device was bent or unbent at regular intervals. Cheng et al. evaluated the energy generated by blood circulation from the viability and effectiveness of a PVDF film-based implantable PENG, as schematically shown in Figs. 3(e)–(g). When implanted into the rat body, the induced charge distributions onto the device in the expanded and contracted states reflect the systolic BP and Diastolic BP contrasted voltage peaks. Specifically, the positive peaks are due to systolic BP, while diastolic represents a negative voltage peak in Fig. 3(h), [20]. The electrical output of the P(VDF-TrFE) nanofibers in the wound area were studied by Wang et al., and Sprague Dawley (SD) rat legs were implanted with polarized P(VDF-TrFE) scaffolds. A photograph of implanted P(VDF-TrFE) nanofiber scaffolds in the subcutaneous thigh region (upper right) of an SD rat is shown in Fig. 3(i). The corresponding output voltage produced by the device showed similar peak flow pressure variations in the in-vitro and invivo experiments [20]. Figure 3j represents the MTT assay analysis performed using fibroblast cells [21]. Based on the study by Sun et al., Fig. 3(k) represents the schematics of the multilayered stretchable sensors with the enlarged electrode patterns for the curved surfaces such as knee joints, stomachs, and pig hearts, along with the initially attached devices and then the expected deformation of the devices after a certain period. The scale bar was 1 cm for the patchable sensors Fig. 3(l) [22].
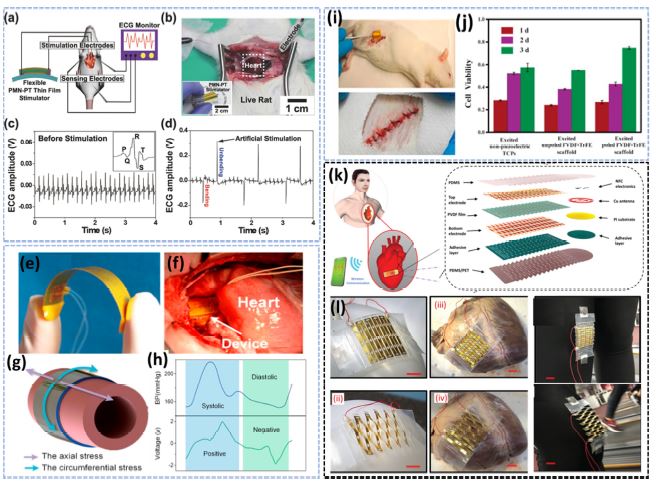
Stretchable PMN-PT thin film self-powered cardiac pacemaker. (a) An experimental system diagram for a flexible PMN-PT thin-film-powered simulated cardiac pacemaker (b) Flexible PMN-PT heart stimulator is used to photograph the animal experiment that stimulates the beating heart. (c) The evaluated ECG through artificial stimulation. (d) The artificial peaks on the ECG are made by the ordinary motion of bending and unbending of the flexible PEH Ref. [19], (e) PEH is based on a polarized PVDF thin film. (f) Image of the highly flexible device design at the implantation site and the device wrapped around the pig ascending aorta. (g) An enlarged schematic of the aorta wall shows axial and circumferential stress distribution. (h) Comparison of the blood pressure (BP) waveform with the output voltage of the implanted device. Ref. [20]. (i) An illustration of the implantation of electrospun P(VDF-TrFE) nanofiber scaffolds. (j) MTT assay analysis, production of fibroblast cells on tissue culture Ref. [21], (k) Schematic illustration of a self-powered sensing device and system with a radio communication interface. (l) Implement the flexible sensors on different surfaces, such as balloons, pig hearts, and stifle joints, the sensor's initial and deformed states of the sensor Ref. [22].
5. SELF-POWERED PATCHABLE AND WEARABLE PENG SENSORS
Yang et al. achieved real-time monitoring of physiological signals based on three-dimensional, hierarchically interlocked PVDF/ZnO pressure sensors. The sensors were conformally adhered to the calf muscles and attached to the wrist and chest (Figs. 4(a) and (b)) [23]. Sensors implanted on calf muscles can help diagnose Parkinson's disease (PD). Fig. 4(c) shows a graphical representation of the ratio of the electrical signals from the left leg to those from the right leg during walking. Variations in the R values can help recognize different gait patterns [23]. Fig. 4 (d) shows a schematic of the multistage self-powered sensation matrix along with the enlarged sensing pixels for detecting complex human body motions. Figs. 4 (e) and (f) show the four-column multiple sensors attached to the palm to capture the lifting and bending motions of the hand and the corresponding electrical output signal. Graphene was used as the electrode material for each sensor [24]. Fig. 4(g) depicts a porous, structural, P(VDF-TrFE)-fiber mat-based self-powered flexible device for detecting finger movement, regardless of the finger motion whether unbendable or flexible. Fig. 4(h) shows the electrical output signals generated by the sensors when fingers were moved along the word “THANK YOU” appearing on the screen [25].
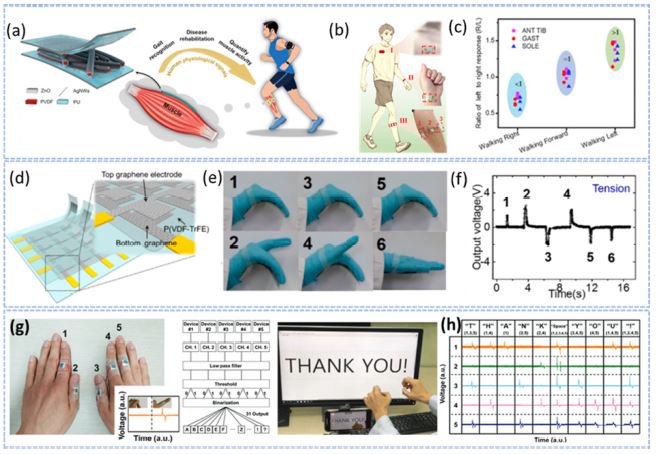
PENG for human motion sensing (a) Schematic of a three-dimensional, hierarchically entangled PVDF/ZnO fiber-based PENG sensor to monitor muscle movement. (b) Schematic of the piezo sensors attached to the chest (I), wrist (II), and three calf muscles (III). (c) The right leg's calf muscle’s response amplitude relative to that of the left when walking in various directions. Ref. [23]. (d) Schematic of the sensory matrix with multiple stages. (e) Photograph of the four-column sensors placed in the palm to record the bending and raising motions of the hand and (f) the related sensing signals Ref. [24]. (g) Photograph of five fingers with motion sensors attached; The inset shows the voltage output during finger bending and unbending motions, and the logical flowchart of the LabVIEW program used to recognize finger movements and translate the signal into an output message. (h) A screenshot of the result and the voltage signal for each character Ref. [25].
A self-powered 2D material-based sensor is shown in Fig. 5(a), along with an optical microscopy image of a typical MoSe2PENG. The device was attached to the index finger and wrist, as shown in Figs. 5(b) and (c) [26]. The piezo response of the sensors increased when the finger and wrist were bent from 30° to 90°. Fig. 5(d) represents the graphical abstract for sensing daily human activities with electrospun PVDF-TrFE/MXene composites with excellent piezoelectric properties and a linear pressure-electrical signal response [26]. This sensor is humidity-sensitive when affixed under the foot and can detect foot sweating, as shown in Figs. 5(f) and (g) [27]. However, the performance of the sensors decreases with prolonged walking and sweating. Joint movements exhibit a direct relationship with electrical signals when sensors are implanted. Fig. 5(h) shows the electrical signals obtained from the stretching and closing-into-a-fist hand movements with varying hand strength. Fig. 5(i) shows the piezoelectric signals generated by neck movement in different directions. The device attached to the neck has top and bottom sensors that produce complementary signals when moved in opposite directions [28, 29].
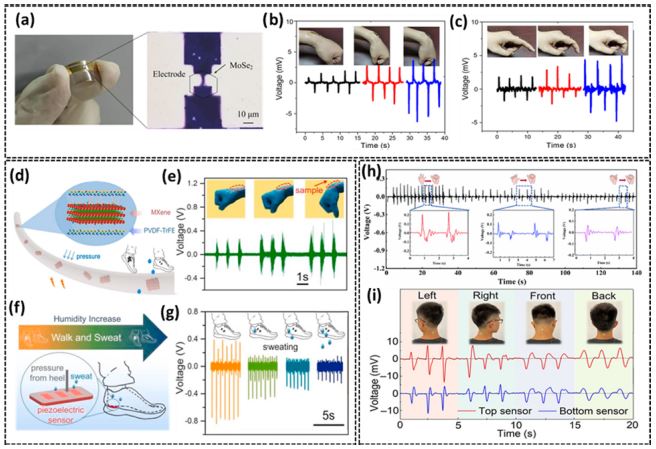
(a) Photograph of the MoSe2 PENG and its optical microscopy image. (b) and (c) MoSe2 nanogenerators based mechanical energy harvested from the wrist and finger joint Ref. [26]. (d) Graphical representation and three-dimensional molecular model of the β-phase formation during the electrospinning procedure. (e) Voltage changes of the composite film attached to the wrist when the wrist bent at different angles. (f) A schematic representation of the sweat sensors attached beneath the heel detects foot perspiration. (g) Voltage signals when the foot sweats and pressure is applied from the heel Ref. [27]. (h) Voltage signals in reaction to the hand being extended out or compressed to various degrees of strength. Enlarged images of various signals are displayed in the inset Ref. [28]. (i) Piezoelectric signals produced by neck movements Ref. [29].
6. CONCLUSIONS
This review summarizes the recent development and performance enhancement of PENGs and their advancements in mechanical energy harvesting and self-powered multi-operational sensors. Many research fields benefited from the invention of PENGs considering their energy harvesting to self-powered systems. Until now, significant advancements in PENGs and self-powered sensors have been made. Nevertheless, there are challenges for advancements in the future. In conclusion, stretchable, flexible piezoelectric energy harvesters through strong piezoelectricity have shown a significant potential to sustain the long-term functionality of wearable and bio implantable devices. Furthermore, animal in vivo investigations show that electrical output derived from the motion of internal organs is adequate for implanted devices with low power requirements, such as pacemakers and blood pressure monitors. To create flexible and stretchable PENGs and self-powered sensors, it is now necessary to use three essential technologies: (1) innovative material synthesis, (2) stretchable mechanical designs, and (3) improved fabrication techniques.
Acknowledgments
This work was financially supported by Nano Material Technology Development Program (2020M3H4A1A03084600) and Basic Science Research Programs (2021R1A2C2010990) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT.
References
-
Z. L. Wang and J. Song, “Piezoelectric nanogenerators based on zinc oxide nanowire arrays”, Sci., Vol. 312, No. 5771, pp. 242-246, 2006.
[https://doi.org/10.1126/science.1124005]

-
Z. Zhao, Y. Dai, S. X. Dou, and J. Liang, “Flexible nanogenerators for wearable electronic applications based on piezoelectric materials”, Mater. Today Energy, Vol. 20, p. 100690. 2021.
[https://doi.org/10.1016/j.mtener.2021.100690]

-
S. Lee, S. H. Bae, L. Lin, Y. Yang, C. Park, S. W. Kim, S. N. Cha, H. Kim, Y. J. Park, and Z. L. Wang, “Super-flexible nanogenerator for energy harvesting from gentle wind and as an active deformation sensor”, Adv. Funct. Mater., Vol. 23, No. 19, pp. 2445-2449, 2013.
[https://doi.org/10.1002/adfm.201202867]

-
W. Han, H. He, L. Zhang, C. Dong, H. Zeng, Y. Dai, L. Xing, Y. Zhang, and X. Xue, “A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays”, ACS Appl. Mater. Interfaces, Vol. 9, No. 35, pp. 29526-29537, 2017.
[https://doi.org/10.1021/acsami.7b07990]

-
M. Du, D. Zhang, W. Fan, K. Zhao, Y. Xia, Z. Nie, and K. Sui, “Ionic diode-based self-powered ionic skins with multiple sensory capabilities”, Mater. Today Phys., p. 100744, 2022.
[https://doi.org/10.1016/j.mtphys.2022.100744]

- D. C. Han, H. J. Shin, S. H. Yeom, and W. Lee, “Wearable human health-monitoring band using inkjet-printed flexible temperature sensor”, J. Sens. Sci. Technol., Vol. 26, No. 5, pp. 301-305, 2017.
- C. I. Kim, T. H. Kwon, S. Y. Yeo, J. S. Yun, Y. H. Jeong, Y. W. Hong, J. H. Cho, and J. H. Paik, “Study of Broadband Piezoelectric Harvester using the Bender-Type Module”, J. Sens. Sci. Technol., Vol. 27, No. 2, pp. 112-117, 2018.
-
Y. Jung and H. Cho, “Flexible Pressure Sensors Based on Three-dimensional Structure for High Sensitivity”, J. Sens. Sci. Technol., Vol. 31, No. 3, pp. 145-150. 2022.
[https://doi.org/10.46670/JSST.2022.31.3.145]

-
J. Kim, J. H. Lee, H. Ryu, J. H. Lee, U. Khan, H. Kim, S. S. Kwak, and S. W. Kim, “High-performance piezoelectric, pyroelectric, and triboelectric nanogenerators based on P (VDF-TrFE) with controlled crystallinity and dipole alignment”, Adv. Funct. Mater., Vol. 27, No. 22, p. 1700702, 2017.
[https://doi.org/10.1002/adfm.201700702]

-
S. A. Han, T. H. Kim, S. K. Kim, K. H. Lee, H. J. Park, J. H. Lee, and S. W. Kim, “Point-defect-passivated MoS2 nanosheet-based high performance piezoelectric nanogenerator”, Adv. Mater., Vol. 30, No. 21, p. 1800342, 2018.
[https://doi.org/10.1002/adma.201800342]

-
D. Wang, D. Zhang, P. Li, Z. Yang, Q. Mi, and L. Yu, “Electrospinning of flexible poly (vinyl alcohol)/MXene nanofiber-based humidity sensor self-powered by monolayer molybdenum diselenide piezoelectric nanogenerator”, Nanomicro lett., Vol. 13, No .1, pp. 1-13, 2021.
[https://doi.org/10.1007/s40820-020-00580-5]

-
S. N. Cha, J. S. Seo, S. M. Kim, H. J. Kim, Y. J. Park, S. W. Kim, and J. M. Kim, “Sound-driven piezoelectric nanowire-based nanogenerators”, Adv. Mater., Vol. 22, No. 42, pp. 4726-4730, 2010.
[https://doi.org/10.1002/adma.201001169]

-
S. Ahn, Y. Cho, S. Park, J. Kim, J. Sun, D. Ahn, M. Lee, D. Kim, T. Kim, H. Shin, and J. J. Park, “Wearable multimode sensors with amplified piezoelectricity due to the multi local strain using 3D textile structure for detecting human body signals”, Nano Energy, Vol. 74, p. 104932, 2020.
[https://doi.org/10.1016/j.nanoen.2020.104932]

-
D. Y. Park, D. J. Joe, D. H. Kim, H. Park, J. H. Han, C. K. Jeong, H. Park, J. G. Park, B. Joung, and K. J. Lee, “Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors”, Adv. Mater., Vol. 29, No. 37, pp. 1702308, 2017
[https://doi.org/10.1002/adma.201702308]

-
Z. Huo, X. Wang, Y. Zhang, B. Wan, W. Wu, J. Xi, Z. Yang, G. Hu, X. Li, and C. Pan, “High-performance Sb-doped p-ZnO NW films for self-powered piezoelectric strain sensors”, Nano Energy, Vol. 73, p. 104744, 2020.
[https://doi.org/10.1016/j.nanoen.2020.104744]

-
E. J. Lee, T. Y. Kim, S. W. Kim, S, Jeong, Y. Choi, and S. Y. Lee, “High-performance piezoelectric nanogenerators based on chemically reinforced composites”, Energy Environ. Sci., Vol. 11, No. 6, pp. 1425-1430, 2018.
[https://doi.org/10.1039/C8EE00014J]

-
X. Chou, J. Zhu, S. Qian, X. Niu, J. Qian, X. Hou, J. Mu, W. Geng, J. Cho, J. He, and C. Xue, “All-in-one filler-elastomer-based high-performance stretchable piezoelectric nanogenerator for kinetic energy harvesting and self-powered motion monitoring”, Nano Energy, Vol. 53, pp. 550-558, 2018.
[https://doi.org/10.1016/j.nanoen.2018.09.006]

-
V. Vivekananthan, A. Chandrasekhar, N. R. Alluri, Y. Purusothaman, W. J. Kim, C. N. Kang, and S. J. Kim, “A flexible piezoelectric composite nanogenerator based on doping enhanced lead-free nanoparticles”, Mater. Lett., Vol. 249, pp. 73-76, 2019.
[https://doi.org/10.1016/j.matlet.2019.02.134]

-
G. T. Hwang, H. Park, J. H. Lee, S. Oh, K. I. Park, M. Byun, H. Park, G. Ahn, C. K. Jeong, K. No, and H. Kwon, “Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester”, Adv. Mater., Vol. 26, No. 28, pp. 4880-4887, 2014.
[https://doi.org/10.1002/adma.201400562]

-
X. Cheng, X. Xue, Y. Ma, M. Han, W. Zhang, Z. Xu, H. Zhang, and H. Zhang, “Implantable and self-powered blood pressure monitoring based on a piezoelectric thin film: Simulated, in vitro and in vivo studies”, Nano Energy, Vol. 22, pp. 453-460, 2016.
[https://doi.org/10.1016/j.nanoen.2016.02.037]

-
A. Wang, Z. Liu, M. Hu, C. Wang, X. Zhang, B. Shi, Y. Fan, Y. Cui, Z. Li, and K. Ren, “Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing”, Nano Energy, Vol. 43, pp. 63-71, 2018.
[https://doi.org/10.1016/j.nanoen.2017.11.023]

-
R. Sun, S. C. Carreira, Y. Chen, C. Xiang, L. Xu, B. Zhang, M. Chen, I. Farrow, F. Scarpa, and J. Rossiter, “Stretchable piezoelectric sensing systems for self-powered and wireless health monitoring”, Adv. Mater. Technol., Vol. 4, No. 5, p. 1900100, 2019.
[https://doi.org/10.1002/admt.201900100]

-
T. Yang, H. Pan, G. Tian, B. Zhang, D. Xiong, Y. Gao, C. Yan, X. Chu, N. Chen, S. Zhong, and L. Zhang, “Hierarchally structured PVDF/ZnO core-shell nanofibers for self-powered physiological monitoring electronics”, Nano Energy, Vol. 72, p. 104706, 2020.
[https://doi.org/10.1016/j.nanoen.2020.104706]

-
Q. Zhang, T. Jiang, D. Ho, S. Qin, X. Yang, J. H. Cho, Q. Sun, and Z. L. Wang, “Transparent and self-powered multistage sensation matrix for mechanosensation application”, ACS Nano, Vol. 12, No. 1, pp. 254-262, 2018.
[https://doi.org/10.1021/acsnano.7b06126]

-
S. Lee, D. Kim, S. Lee, Y. I. Kim, S. Kum, S. W. Kim, Y. Kim, S. Ryu, and M. Kim, “Ambient Humidity-Induced Phase Separation for Fiber Morphology Engineering toward Piezoelectric Self-Powered Sensing”, Small, Vol. 18, No. 17, p. 2105811, 2022.
[https://doi.org/10.1002/smll.202105811]

-
P. Li and Z. Zhang, “Self-powered 2D material-based pH sensor and photodetector driven by monolayer MoSe2 piezoelectric nanogenerator”, ACS Appl. Mater. Interfaces, Vol. 12, No. 52, pp. 58132-58139, 2020.
[https://doi.org/10.1021/acsami.0c18028]

-
S. Wang, H. Q. Shao, Y. Liu, C. Y. Tang, X. Zhao, K. Ke, R. Y. Bao, M. B. Yang, and W. Yang, “Boosting piezoelectric response of PVDF-TrFE via MXene for self-powered linear pressure sensor”, Compos. Sci. Technol., Vol. 202, p. 108600, 2021.
[https://doi.org/10.1016/j.compscitech.2020.108600]

-
X. Hou, S. Zhang, J. Yu, M. Cui, J. He, L. Li, X. Wang, and X. Chou, “Flexible Piezoelectric Nanofibers/Polydimethylsiloxane-Based Pressure Sensor for Self-Powered Human Motion Monitoring”, Energy Technol., Vol. 8, No. 3, p. 1901242, 2020.
[https://doi.org/10.1002/ente.201901242]

-
Y. Hong, B. Wang, W. Lin, L. Jin, S. Liu, X. Luo, J. Pan, W. Wang, and Z. Yang, “Highly anisotropic and flexible piezoceramic kirigami for preventing joint disorders”, Sci. Adv., Vol. 7, No. 11, p. eabf0795, 2021.
[https://doi.org/10.1126/sciadv.abf0795]
