
Current Development in Bio-implantable Sensors
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Flexible and wearable sensing technologies have emerged as a result of developments in interdisciplinary research across several fields, bringing together various subjects such as biology, physics, chemistry, and information technology. Moreover, various types of flexible wearable biocompatible devices, such customized medical equipment, soft robotics, bio-batteries, and electronic skin patches, have been developed over the last several years that are extensively employed to monitor biological signals. As a result, we present an updated overview of new bio-implantable sensor technologies for various applications and a brief review of the state-of-the-art technologies.
Keywords:
Bio-implantable, Biomedical, Flexible, Sensors1. INTRODUCTION
Flexible bio-implantable devices emphasize device flexibility and offer specific traits and benefits, such as low weight, high elasticity, and the ability to be miniaturized. They have triggered a fresh wave of technical advancement in the biomedical device industry by being widely used in the fields of biomedicine, data collection, human-machine interfaces, and robotics [1-3]. Bio-implantable technologies are used in the human body to accomplish real-time monitoring of bodily information, personalized diagnoses, and therapy, although there is sufficient scope for advancement. Individualized healthcare gadgets that may be used in households can provide factual information regarding human physiological issues, lowering the need for hospital care and conserving resources. The general population is becoming more familiar with bio-implantable products [4]. The Xinsijie Industrial Research Centre published an industry study report that estimated that the worldwide biosensor market would increase to USD 23.62 billion by 2021. Moreover, the worldwide biosensor market is anticipated to develop at an average yearly growth rate of almost 9% between 2022 and 2026. Currently, smart watches, sports wristbands, hearing aids, and eyeglasses are among the most commonly used wearable technology. Importantly, these wearable technologies are crucial for real-time physiological monitoring, supplemental therapy, and healthcare industries [5-7]. As society progresses, flexible bio-implantable technology will gain popularity. The above devices have achieved number of co and inter data gathering due to their diverse degrees of sensitivity, lowest detection levels, and ranges. They can also maintain impressive results in difficult situations. These performance improvements have enhanced the reliability, efficiency, and precision of sensing weak signals in the biological system. Various in vivo sensor devices have also emerged as a result of the development of implantable, adaptable, and biodegradable materials [8-10]. The minimum detection threshold of a sensor is referred to as its sensitivity, and it relates to the strongest signal that may be recognized and measured. The response and relaxation times influence the ability of the bio-implantable device to quickly respond, while the detecting range pertains to the sensing signal that may be utilized. Importantly, the applicability and sensing capability of a wearable device are determined by these crucial criteria. In addition, the sensor’s robustness, cycle stability, and biocompatibility are crucial for the long-term stability of wearable technologies. Moreover, these qualities are frequently viewed as fundamental elements of flexible sensor devices [11]. It is worth noting that flexible wearable sensors for biomedicine often have low quality, although they have good flexibility and adaptability. Complicated body surfaces can be accommodated owing to this great flexibility, and the function of the device is unaffected by sensor deformation because of various limb motions. High compliance is a crucial aspect of implanted sensors since it can prevent any adverse consequences, such allergy and inflammation, between both the device and the organism. Having low quality makes the device portable and heightens people’s feeling of experience, which is a crucial sensor production indication. Bio-implantable sensors for healthcare still have drawbacks, and their high production costs make it challenging to sell them. There are several dispensable wearable sensors available, although these can pollute the environment. Current research on wearable devices focus on cost reduction, recycling, and post-use sensor deterioration. For continued advancement, this area requires a thorough review.
In this paper, we provide a concise summary of recent breakthroughs in bio-implantable sensors. First, we discuss the development of flexible materials and their outstanding electrochemical performance. Then, we discussed the uses of this technology in the human body. Finally, in the last section, we summarize the opportunities and challenges associated with flexible bio-implantable device technologies.
2. MATERIALS
The materials selected and how the sensors are manufactured can have a significant impact on how well they work. Moreover, substantial requirements need to be met regarding the materials’ flexibility, conductivity, and durability in bio-medically focused sensor systems [12]. In this section, we provide a brief overview of the various materials that have been used to fabricate these types of sensors.
2.1 Polymers
Polymeric materials are frequently employed as flexible substrates and coating materials to enhance the performance of sensors during the manufacturing process. Additionally, doping and altering polymer materials have allowed the development of novel organizational structures and distinguishing characteristics, such as increased conductivity and ion mobility, in addition to more robust structural and mechanical features [13-15]. A nonstandard phenolic polymer with potent underwater adsorption was described by Ejima et al., where adhesion capability was improved by adding a hydroxyl group to the catechol moiety [16]. Tao et al. described a wet-woven ultrafine polyaniline fiber utilizing a solvent-exchange technique. The polymer fiber decreased the viscosity of the fiber by solvent diffusion while increasing the tensile capacity of the fiber (Fig. 1) [17]. Polymer fiber is a superior electrode material that conducts current and stores energy better than certain carbon nanostructures. Moreover, its electrochemical performance stability is anticipated to represent a significant advancement in the capacity of a system to feed itself [18]. With a focus on bio-implantable textiles and sensor integration, these polymer materials were developed to suit a wide range of specifications. Additionally, the functionalization, refining, and fibrosis of biomedical polymer materials are rapidly advancing [19]. Synthesized polymers are extensively employed and have achieved outstanding outcomes in implantable, biodegradable, catalyzed, energy storage, and energy conversion applications. Nevertheless, it is difficult to change the intricacy of polymer material synthesis and lower the expenses associated with it. Therefore, effective elimination of these limitations would significantly aid in the growth of research findings. Polyethylene terephthalate (PET) is frequently utilized in flexible printed circuit boards (PCB) since it has good mechanical characteristics. Given this property, PET is employed as a device encapsulating layer and simultaneously provides great electrical insulating qualities.
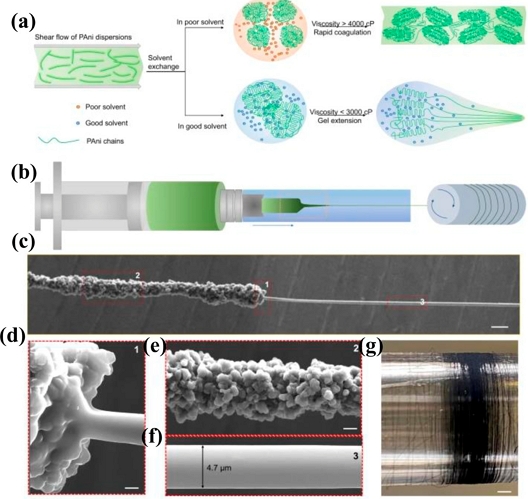
(a) Schematic of a good solvent exchange strategy for preparing ultrafine polyaniline fibers (UFPFs) in a modified wet spinning protocol; (b) schematic of modified wet spinning process; (c) scanning electron microscope (SEM) image of the marked region in (b), showing the sharp necking behavior of gel polyaniline fibers. The close observation of region 1 (d), region 2 (e), and region 3 (f) in the marked zone of (c); (g) photograph of a 5.4-km-long UFPF collected in 2 h. Scale bars: (c) 20μm, (d) 2μm, (e) 10μm, and (g) 150mm (reproduced from [17], with permission from Springer Nature, 2022).
Additionally, PET may transmit light and be used as a protective coating in photo sensors. Polyvinyl alcohol (PVA) is widely applied in device adhesives and synthetic fibers and is widely used in the production of disposable devices because it has good film-forming capabilities and is easily biodegradable. Polyethylene naphthalate (PEN) is frequently utilized in capacitor films and flexible PCBs. It is accessible as a film and is superior to PET in terms of mechanical durability and heat resistance when used as a device component. Moreover, it performs well in preventing the passage of gas and water and can be used to package gadgets. Compared to PET materials, polyimide (PI) offers few advantages (e.g., superior heat resistance). In addition, it is unchanged by temperature or humidity and possesses great environmental stability. Consequently, PI can be used as a shield over sensors to keep undetectable stimuli from impairing the functionality of the apparatus. The flexible substance that is most frequently utilized is polydimethylsiloxane (PDMS). It is a force-sensitive material with exceptional flexibility that works well in both high- and low-temperature settings. Additionally, PDMS has excellent dielectric properties and may be used as a substrate for pressure sensors. As a result, it is employed in applications for pressure sensing, flexible wearables, and gadget wrapping. Moreover, PDMS has a certain degree of air permeability. Although PET, PI, and PEN are not sufficiently malleable for use in flexible devices, their flexibility can be increased by decreasing their thickness. Wearable sensors that take the form of patches commonly employ PVA and PDMS.
2.2 Hydrogels
Small, flexible gadgets should be more sophisticated and compatible to replace conventional medical devices. The invention of individualized hydrogels has increased the potential of medical instruments that are used in human-machine interfaces. Hydrogels are exceptionally biocompatible as a result of dissolving, reactivity, and modification, providing them with numerous beneficial features [20-22]. For instance, Kim et al. devised a thin, low-impedance, ultra-permeable, and ultra-soft hydrogel that may act as a liquid electrolyte on skin and create a quasi-solid interface with tissue-like properties (Fig. 2) [23].
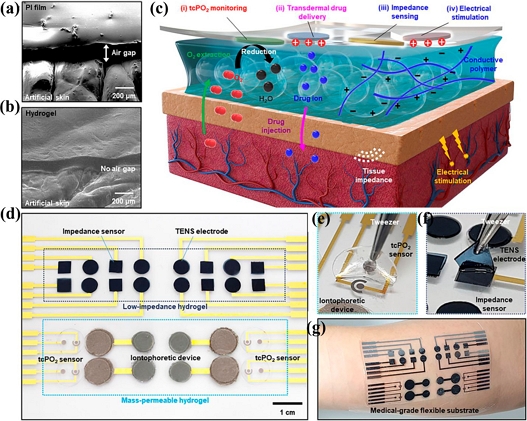
(a) and (b) SEM images of PI film (a) and tissue-like ultrasoft hydrogel (b) mounted on artificial skin; (c) illustration of the structure, requirement, and roles of the hydrogel interface between human skin and wearable bioelectronics; (d) photograph of an integrated wearable device on a flexible substrate; (e) photograph of mass-permeable hydrogel on a tcPO2 sensor; (f) photograph of low-impedance hydrogel on a TENS electrode; (g) photograph of an integrated device applied to human skin (reproduced from [23], with permission from Science, 2021).
Wang et al. proposed to establish a symmetrical interface between both the hydrogel and the anatomical sites to adhere hydrogels to various solid surfaces. This method has several uses in bioassays and healthcare and enables hydrogels to be naturally attached to human tissues [24]. Qiu et al. devised a highly soluble, hyper branched nanoparticle-incorporated polymeric hydrogel with remarkable flexibility and stretchability. This hydrogel possesses no short-term entanglement, fatigue-free features, and hysteresis-free material characteristics under cyclic loading [25]. High device performance in locations where recurrent human movements take place requires the least amount of material energy dissipation (e.g., heartbeat, breathing, and movement). The primary cross-linked, which significantly expanded polymer chains in hydrogel systems, are hyper branched nanoparticles. Moreover, they have outstanding elastic characteristics and can be connected without temporary entanglement, making it promising for strain and impedance sensors [26, 27]. The tendency of the hydrogel medium to expand makes the network voids that result, which is beneficial for the uptake and transportation of macromolecules. Nonetheless, hydrogels can be expensive due to costly polymer components and integration can be more challenging. Finally, it is evident that hydrogels significantly perform and are affordable, despite their small cost and yield deficiencies.
2.3 Carbon-based materials
Researchers have afforded extensive attention to carbon nanomaterials, such as graphene, carbon nanotubes, and MXene, due to their exceptional electrical conductivity, superior performance indicators, and distinctive nanostructures. Since its discovery more than ten years ago, graphene has demonstrated outstanding functionality and a great carrying ability when combined with various proteins due to its extremely large lateral space and good electrical conductivity [28, 29]. Carbon nanotubes and MXene have an inherent benefit in terms of the hybridization of carbon-based compounds because of their excellent biocompatibility, inherent biodegradability of carbon-based compounds, and complementing conductivity. In contrast, MXene is more likely to exhibit a variety of biological effects due to its hydrophilic nature, diffusivity, and controllability formed by functional groups. Moreover, carbon nanoparticles are particularly competitive in device cycle testing due to their exceptional stability [30].
3. BIO-IMPLANTABLE SENSORS
Flexible, bio-implantable sensor devices are used in human-machine interfaces, healthcare, and specialized diagnosis and treatment technologies to determine the physiological state of the body. The manufacture of devices has been simplified by using high-precision preparation techniques, including 3D printing, photolithography, and printed coating techniques, which have significantly improved accuracy and yield [31, 32]. Sensors can be divided into two categories based on the detection site: in vitro and in vivo. Furthermore, in vitro sensors are divided in electronic skin classes as well as other medical equipment depending on the package design and purpose. Although many researchers have established various approaches and solutions, the choice of tools for in vivo sensing is more challenging than for in vitro sensors [33, 34].
3.1 In vitro sensors
Wearable in vitro flexible devices that can measure the movement of different body parts help with illness diagnosis and provide a greater degree of human-machine connection. Additionally, they are a prominent area of focus for in vitro sensing [35]. Medical equipment and electronic skin are the two most common types of in vitro sensor devices. As a result, flexible wearable technology has strong bending and stretching capability and can adapt to human limb motions. The majority of human surfaces can be used as an effective integrated platform for electronic skin, which has a thin and soft interface. Electronic skin can detect internal physiological data of the human body, making wise decisions and detecting and monitoring illnesses. Nevertheless, built-in sensors must be precise, and the electronic skin must be robust, due the inherent fragility and endurance of human impulses [36]. Deng et al. proposed a patch-type piezoresistive sensor with outstanding resolution and a wide sensing range for tracking user physiological parameters. They employed a multilayered microstructure with polyaniline/polyvinylidene fluoride nanofiber films on the top and bottom layers and interconnecting electrodes with a conical shape inside the center, which was influenced by the architecture of a rose petal. Excellent mechanical flexibility and electrical qualities are demonstrated by the sensor (Fig. 3) [37].
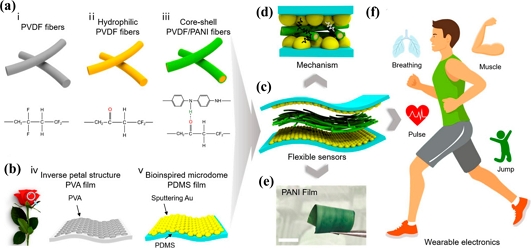
Fabrication process, mechanism, and optical image of designed flexible wearable pressure sensors based on polyvinylidene fluoride/polyaniline nanofibers. (a) Schematic preparation of hierarchical polyaniline nanofibers (i–iii); (b) diagram illustrating the fabrication procedure of the microdome structure inspired by rose petals (iv–v); (c) schematic illustration of the structure design of a flexible pressure sensor; (d) working mechanism of the fabricated piezoresistive sensor; (e) optical photograph of the PANI nanofibers film. Scale bar, 5 mm. (f) Diversified applications that can be enabled by the fabricated sensor (reproduced from [37], with permission from ACS Publications, 2021).
Wen and colleagues developed an electronic skin that can independently sense temperature and pressure by converting pressure into a voltage difference across polar plates [38]. A tapered microstructure is employed by the sensor, which is similar to sensitive pressure detection. On the other hand, the sensor employs a tapered-built triboelectric nanogenerator (TENG) that is also applied to the triboelectric surface. In particular, this electronic skin can sense pressure while separately displaying the temperature signal. The thermoelectric coupling effect can be demonstrated by utilizing a specifically created thermocouple membrane to detect the temperature signal [39]. A stand-alone healthcare patch that resembles the skin and employs a direct interface with the wrist to detect fluctuations in heart rate was presented by Yun et al, which is worth noting. A flexible light-volume tracking sensor array comprising an organic photodiode (OPD) and two organic light-emitting diodes (OLEDs), which make up the body of the patch [40]. Recently, a smart wearable sensing system that integrates TENGs with deep learning technology have been introduced by Wang et al. This system has a significant practical utility in the construction of bionic prostheses and the cognitive learning of visually impaired patients [41]. Wang et al. created a stretchy, wearable, self-driving sensor and the associated system. Extremely low hysteresis and considerably high durability are characteristics of TENGs with grating designs (over 100000 cycles). The triboelectric layer is squeezed to provide an electrical signal when the sensor is twisted or stretched [42]. Wearable and flexible medical gadgets communicate with data through embedded sensors. Compared to electronic skin, medical equipment can have sensors with a range of geometrical and more diversified shapes and a greater amount of sensing data, all of which promise to enhance diagnosis and therapy [43]. A flexible gripper that can hold microbes and sense temperature and pressure was described by Koh et al. [44]. Wang et al. designed a sensor array for lumbar degenerative illnesses to assess the distribution of plantar pressure [45]. In comparison, Wu et al. used a neural network to evaluate and decode a flexible mortise and tenon interlocking construction system's six-dimensional force sensor [46]. Researchers have recommended “non-force” testing in addition to conventional force tests [47]. A fiber-optic temperature sensor based on photoelectric up conversion was described in detail most recently by Ding et al. This sensor showed significant temperature dependence from the transformation of infrared light to visible light [48]. A flexible, inexpensive, quickly manufactured, and ink-based printing humidity sensor was described by Tokito et al. Here, the conductive ink comprised of carbon black and cellulose nanofibers and its resistance is altered in response to variations in humidity. Moreover, the electrical resistance increased as a result of the destruction of the carbon black’s conductive route via the hygroscopic expansion of nanofibers. This hygroscopic property is further enhanced by the porous physical structure of the nanofibers [49].
3.2 In vivo sensors
Individual differences in the mechanical and biological characteristics of human tissue present significant challenges for implanted devices to achieve rapid detection. These challenges are as follows. First, using the current electrical equipment for 3D organs may be challenging because the body may completely disregard them. Second, devices inside the body are within a complex environment, making long-term monitoring dangerous. Third, preserving the quality factor of the RLC circuit while achieving steady transmission utilizing passive wireless reading devices for strain sensors is difficult [50]. Additionally, the complicated physiologic conditions within the body, the production of stomach acid and catabolic enzymes, and the normal compression and extension motions of organs and tissues might impair both the design of the device and the human body. Considering this, Lee et al. presented a suture-process-connected, wireless capacitive fiber optic strain sensor for connective tissue surveillance. Here, the sensing system comprises a passive RLC circuit and a hollow double-helix structure made of two stretchy conductive fibers [50]. Similarly, Jeong et al. suggested an implantable, wirelessly charged soft optoelectronic gadget that can rapidly adapt to changing circumstances. This device is extensively employed for in vivo neuroscience research. The technology uses optoelectronic nerve sensors to do light stimulus, wireless transmission via coil antenna inductive coupling, and wireless charging using radio-frequency energy [51]. Wang et al. demonstrated intraoperative cardiovascular care using ambient pressure as an electrical impulse and a biodegradable, self-fed pressure sensor (Fig. 4) [52].
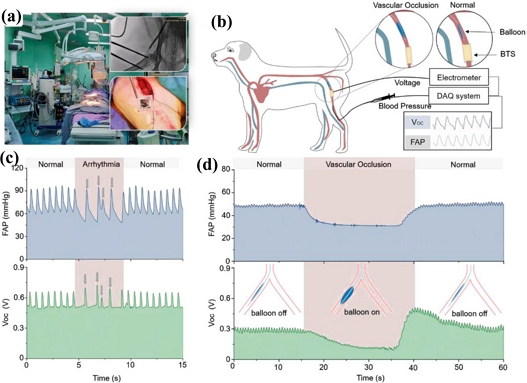
Abnormal cardiac event identification in a large animal. (a) Image of the human-scale animal cardiac monitoring experiment. (b) Schematic of in vivo experimental electrical characterization and physiological signal monitoring. (c) Representative blood pressure that indicates arrhythmia events corresponding to the output signal of the BTS. (d) Simulated abnormal vascular occlusion events detected by the BTS (reproduced from [52], with permission from Wiley, 2021).
Rogers et al. developed a wireless implanted micro flow sensing device that offers steady and dependable flow sensing that provides a readout on a mobile terminal. The sensor measures blood flow using a thermistor and heater. It is connected to a small Bluetooth that is placed to the skin for data collection and video representation on a smartphone [53]. Bao et al. looked at a flexible bio interface for brain tissue that was created by laser cutting a metal-complexed polyimide into the network of connected graphene and nanoparticles, which was then covered in an elastomer [54]. Moreover, implanted sensor technology is ideally suited for transporting medications and offers potential tailored drug delivery for localized therapy. This nearly attrition-free pharmacological therapy is a breakthrough clinical treatment method that significantly improves the effectiveness of the treatment.
4. CONCLUSION
We discussed new studies regarding flexible bio-implantable sensing devices for medical applications, focusing on in vivo and in vitro sensing. The capacity to detect human physiological data has improved due to the development of innovative materials and sensing technologies. Moreover, flexible wearable sensors provide a promising advancement in targeted therapy, medication delivery, cell capture, medical prostheses, health monitoring, and aided diagnosis and treatment. However, there are several challenges regarding future employment and product marketing. For instance, the requirements for detecting those that are closely related to the brittleness of human impulses are still not met by existing materials, whether for in vitro or in vivo sensing. Using flexible wearable devices in biomedicine would expand with the efforts and input from various other disciplines. Finally, further research should be conducted on how these applications translate from the laboratory to clinical settings, to real-world situations.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NO. 2022R1A2C2007784) and the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0017011, HRD Program for Industrial Innovation). This research was also supported by the National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2021M3H2A1038042).
REFERENCES
-
Y. Ohm, C. Pan, M. J. Ford, X. Huang, J. Liao, and C. Majidi, “An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics”, Nat. Electron., Vol. 4, No. 3, 185–192, 2021.
[https://doi.org/10.1038/s41928-021-00545-5]

-
Q. Su, Q. Zou, Y. Li, Y. Chen, S.-Y. Teng, J. T. Kelleher, R. Nith, P. Cheng, N. Li, and W. Liu, “A stretchable and strain-unperturbed pressure sensor for motion interference–free tactile monitoring on skins”, Sci. Adv., Vol. 7, No. 48, pp. eabi4563(1)- eabi4563(9), 2021.
[https://doi.org/10.1126/sciadv.abi4563]

-
A. H. Anwer, N. Khan, M. Z. Ansari, S.-S. Baek, H. Yi, S. Kim, S. M. Noh, and C. Jeong, “Recent advances in touch sensors for flexible wearable devices”, Sens., Vol. 22, No. 12, pp. 4460(1)-4460(21), 2022.
[https://doi.org/10.3390/s22124460]

-
K. Guo, S. Wustoni, A. Koklu, E. Díaz-Galicia, M. Moser, A. Hama, A. A. Alqahtani, A. N. Ahmad, F. S. Alhamlan, and M. Shuaib, “Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors”, Nat. Biomed. Eng., Vol. 5, No. 7, pp. 666–677, 2021.
[https://doi.org/10.1038/s41551-021-00734-9]

-
C. Chen, S. Zhao, C. Pan, Y. Zi, F. Wang, C. Yang, and Z. L. Wang, “A method for quantitatively separating the piezoelectric component from the as-received 'Piezoelectric' signal”, Nat. Commun., Vol. 13, No. 1, pp. 1–9, 2022.
[https://doi.org/10.1038/s41467-022-29087-w]

-
H. Xu, W. Zheng, Y. Wang, D. Xu, N. Zhao, Y. Qin, Y. Yuan, Z. Fan, X. Nan, and Q. Duan, “Flexible tensile strain-pressure sensor with an off-axis deformation-insensitivity”, Nano Energy, p. 107384, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107384]

-
Y. Wang, C. Xu, X. Yu, H. Zhang, and M. Han, “Multilayer flexible electronics: Manufacturing approaches and applications”, Mater. Today Phys., p. 100647, 2022.
[https://doi.org/10.1016/j.mtphys.2022.100647]

-
K. Cao, M. Wu, J. Bai, Z. Wen, J. Zhang, T. Wang, M. Peng, T. Liu, Z. Jia, and Z. Liang, “Beyond Skin Pressure Sensing: 3D Printed Laminated Graphene Pressure Sensing Material Combines Extremely Low Detection Limits with Wide Detection Range”, Adv. Funct. Mater., p. 2202360, 2022.
[https://doi.org/10.1002/adfm.202202360]

-
Y. Chen, C. Zhang, R. Yin, A. Yin, Q. Feng, F. Liu, J. Shao, T. Su, H. Wang, and G. Chen, “Environmentally adaptive and durable hydrogels toward multi-sensory application”, Chem. Eng. J., Vol. 449, p. 137907, 2022.
[https://doi.org/10.1016/j.cej.2022.137907]

-
G. Khandelwal and R. Dahiya, “Self-Powered Active Sensing based on Triboelectric Generator”, Adv. Mater.,p. 2200724, 2022.
[https://doi.org/10.1002/adma.202200724]

-
S. Lin, S. Hu, W. Song, M. Gu, J. Liu, J. Song, Z. Liu, Z. Li, K. Huang, and Y. Wu, “An ultralight, flexible, and biocompatible all-fiber motion sensor for artificial intelligence wearable electronics”, Npj Flex. Electron., Vol. 6, No. 1, pp. 1–8, 2022.
[https://doi.org/10.1038/s41528-022-00158-8]

-
Z. Zhao, K. Liu, Y. Liu, Y. Guo, and Y. Liu, “Intrinsically flexible displays: key materials and devices”, Natl. Sci. Rev., Vol. 9, No. 6, p. nwac090, 2022.
[https://doi.org/10.1093/nsr/nwac090]

-
Z. Tang, C. He, H. Tian, J. Ding, B.S. Hsiao, B. Chu, and X. Chen, “Polymeric nanostructured materials for biomedical applications”, Prog. in Polym. Sci., Vol. 60, pp. 86–128, 2016.
[https://doi.org/10.1016/j.progpolymsci.2016.05.005]

-
Z. Wang, H. Cui, S. Li, X. Feng, J. Aghassi-Hagmann, S. Azizian, and P. A. Levkin, “Facile approach to conductive polymer microelectrodes for flexible electronics”, ACS Appl. Mater. Interfaces, Vol. 13, No. 18, pp. 21661–21668, 2021.
[https://doi.org/10.1021/acsami.0c22519]

-
S. Y. Son, G. Lee, H. Wang, S. Samson, Q. Wei, Y. Zhu, W. You, “Integrating charge mobility, stability and stretchability within conjugated polymer films for stretchable multifunctional sensors”, Nat. Commun., Vol. 13, No. 1, pp. 1–11, 2022.
[https://doi.org/10.1038/s41467-022-30361-0]

-
B. Cheng, J. Yu, T. Arisawa, K. Hayashi, J. J. Richardson, Y. Shibuta, and H. Ejima, “Ultrastrong underwater adhesion on diverse substrates using non-canonical phenolic groups”, Nat. Commun., Vol. 13, No. 1, pp. 1–9, 2022.
[https://doi.org/10.1038/s41467-022-29427-w]

-
B. Fang, J. Yan, D. Chang, J. Piao, K. M. Ma, Q. Gu, P. Gao, Y. Chai, and X. Tao, “Scalable production of ultrafine polyaniline fibres for tactile organic electrochemical transistors”, Nat. Commun., Vol. 13, No. 1, pp. 1–9, 2022.
[https://doi.org/10.1038/s41467-022-29773-9]

-
G. Wu, X. Wu, X. Zhu, J. Xu, and N. Bao, “Two-dimensional hybrid nanosheet-based supercapacitors: From building block architecture, fiber assembly, and fabric construction to wearable applications”, ACS Nano, Vol. 16, No. 7, pp. 10130–10155, 2022.
[https://doi.org/10.1021/acsnano.2c02841]

-
K. Dong, X. Peng, R. Cheng, C. Ning, Y. Jiang, Y. Zhang, and Z. L. Wang, “Advances in High-Performance Autonomous Energy and Self-Powered Sensing Textiles with Novel 3D Fabric Structures”, Adv. Mater., p. 2109355, 2022.
[https://doi.org/10.1002/adma.202109355]

-
V. G. Muir and J. A. Burdick, “Chemically modified biopolymers for the formation of biomedical hydrogels”, Chem. Rev., Vol. 121, No. 18, pp. 10908–10949, 2020.
[https://doi.org/10.1021/acs.chemrev.0c00923]

-
J. Yang, J. An, Y. Sun, J. Zhang, L. Zu, H. Li, T. Jiang, B. Chen, and Z. L. Wang, “Transparent self-powered triboelectric sensor based on PVA/PA hydrogel for promoting human-machine interaction in nursing and patient safety”, Nano Energy, Vo. 97, p. 107199, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107199]

-
Z. S. Nishat, T. Hossain, M. N. Islam, H. P. Phan, M. A. Wahab, M. A. Moni, C. Salomon, M. A. Amin, A. A. I. Sina, and M. S. A. Hossain, “Hydrogel Nanoarchitectonics: An Evolving Paradigm for Ultrasensitive Biosensing”, Small, p. 2107571, 2022.
[https://doi.org/10.1002/smll.202107571]

-
C. Lim, Y. J. Hong, J. Jung, Y. Shin, S.-H. Sunwoo, S. Baik, O. K. Park, S. H. Choi, T. Hyeon, and J. H. Kim, “Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels”, Sci. Adv., Vol. 7, No. 19, pp. eabd3716(1)-eabd3716(11), 2021.
[https://doi.org/10.1126/sciadv.abd3716]

-
T. He, A.R. Puente-Santiago, S. Xia, M.A. Ahsan, G. Xu, and R. Luque, “Experimental and Theoretical Advances on Single Atom and Atomic Cluster-Decorated Low-Dimensional Platforms towards Superior Electrocatalysts”, Adv. Energy Mater., Vol. 12, No. 22, pp. 2200493(1)-2200493(28), 2022.
[https://doi.org/10.1002/aenm.202200493]

-
X. Meng, Y. Qiao, C. Do, W. Bras, C. He, Y. Ke, T. P. Russell, and D. Qiu, “Hysteresis-Free Nanoparticle-Reinforced Hydrogels”, Adv. Mater., Vol. 34, Vol. 7, p, 2108243, 2022.
[https://doi.org/10.1002/adma.202108243]

-
X. Zhang, X. Cheng, Y. Si, J. Yu, and B. Ding, “Elastic and highly fatigue resistant ZrO2-SiO2 nanofibrous aerogel with low energy dissipation for thermal insulation”, Chem. Eng. J., Vol. 433, p. 133628, 2022.
[https://doi.org/10.1016/j.cej.2021.133628]

-
J. Kim, G. Zhang, M. Shi, and Z. Suo, “Fracture, fatigue, and friction of polymers in which entanglements greatly outnumber cross-links”, Sci., Vol. 374, No. 6564, pp. 212–216, 2021.
[https://doi.org/10.1126/science.abg6320]

- M. L. Verma, B. Dhanya, R. Saini, A. Das, and R. S. Varma, “Synthesis and application of graphene-based sensors in biology: a review”, Environ. Chem. Lett., pp. 1–24, 2022.
-
A. Kohls, M. Maurer Ditty, F. Dehghandehnavi, and S.-Y. Zheng, “Vertically Aligned Carbon Nanotubes as a Unique Material for Biomedical Applications”, ACS Appl. Mater. Interfaces, Vol. 14,, No. 5, pp. 6287–6306, 2022.
[https://doi.org/10.1021/acsami.1c20423]

-
B. Gaihre, M. A. Potes, V. Serdiuk, M. Tilton, X. Liu, and L. Lu, “Two-dimensional nanomaterials-added dynamism in 3D printing and bioprinting of biomedical platforms: Unique opportunities and challenges”, Biomater., Vol. 284, p. 121507, 2022.
[https://doi.org/10.1016/j.biomaterials.2022.121507]

-
L. Donaldson, “Wearable sweat sensor for healthcare monitoring”, Elsevier, 2022.
[https://doi.org/10.1016/j.mattod.2022.01.011]

-
Y. G. Park, I. Yun, W. G. Chung, W. Park, D. H. Lee, J. U. Park, “High-Resolution 3D Printing for Electronics”, Adv. Sci., Vol. 9, No. 8, p. 2104623, 2022.
[https://doi.org/10.1002/advs.202104623]

-
Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, and M. Chen, “A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring”, Biosens. Bioelectron., Vol. 203, p. 114026, 2022.
[https://doi.org/10.1016/j.bios.2022.114026]

-
G. D. Cha, W. H. Lee, S.-H. Sunwoo, D. Kang, T. Kang, K. W. Cho, M. Kim, O. K. Park, D. Jung, J. Lee, “Multifunctional Injectable hydrogel for in vivo diagnostic and therapeutic applications”, ACS Nano, Vol. 16, No. 1, pp. 554–567, 2022.
[https://doi.org/10.1021/acsnano.1c07649]

-
L. Pan, L. Han, H. Liu, J. Zhao, Y. Dong, and X. Wang, “Flexible sensor based on Hair-like microstructured ionic hydrogel with high sensitivity for pulse wave detection”, Chem. Eng. J., Vol. 450, p. 137929, 2022.
[https://doi.org/10.1016/j.cej.2022.137929]

-
Y. Wang, D. Liu, Y. Zhang, L. Fan, Q. Ren, S. Ma, and M. Zhang, “Stretchable Temperature-Responsive Multimodal Neuromorphic Electronic Skin with Spontaneous Synaptic Plasticity Recovery”, ACS Nano, Vol. 16, No. 5, pp. 8283–8293, 2022.
[https://doi.org/10.1021/acsnano.2c02089]

-
T. Yang, W. Deng, X. Chu, X. Wang, Y. Hu, X. Fan, J. Song, Y. Gao, B. Zhang, and G. Tian, “Hierarchically microstructure-bioinspired flexible piezoresistive bioelectronics”, ACS Nano, Vol. 15, No. 7, pp. 11555–11563, 2021.
[https://doi.org/10.1021/acsnano.1c01606]

-
Y. Chen, H. Lei, Z. Gao, J. Liu, F. Zhang, Z. Wen, and X. Sun, “Energy autonomous electronic skin with direct temperature-pressure perception”, Nano Energy, Vol. 98, p. 107273, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107273]

-
S. Wei, L. Liu, X. Huang, Y. Zhang, F. Liu, L. Deng, E. Bilotti, and G. Chen, “Flexible and Foldable Films of SWCNT Thermoelectric Composites and an S-Shape Thermoelectric Generator with a Vertical Temperature Gradient”, ACS Appl. Mater. Interfaces, Vol. 14, No. 4, pp. 5973–5982, 2022.
[https://doi.org/10.1021/acsami.1c21363]

-
Y. Lee, J.W. Chung, G.H. Lee, H. Kang, J.-Y. Kim, C. Bae, H. Yoo, S. Jeong, H. Cho, and S.-G. Kang, “Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system”, Sci. Adv., Vol. 7, No. 23, pp. eabg9180(1)- eabg9180(10), 2021.
[https://doi.org/10.1126/sciadv.abg9180]

-
X. Wei, B. Wang, Z. Wu, and Z. L. Wang, “Open-Environment Tactile Sensing System: Towards Simple and Efficient Material Identification”, Adv. Mater., p. 2203073, 2022.
[https://doi.org/10.1002/adma.202203073]

-
C. Li, D. Liu, C. Xu, Z. Wang, S. Shu, Z. Sun, W. Tang, and Z. L. Wang, “Sensing of joint and spinal bending or stretching via a retractable and wearable badge reel”, Nat. Commun., Vol. 12, No. 1, pp. 1–11, 2021.
[https://doi.org/10.1038/s41467-021-23207-8]

-
Y. Yao, Y. Chen, K. Wang, N. Turetta, S. Vitale, B. Han, H. Wang, L. Zhang, and P. Samorì, “A robust vertical nanoscaffold for recyclable, paintable, and flexible light-emitting devices”, Sci. Adv., Vol. 8, No. 10, pp. eabn2225(1)-eabn2225(9), 2022.
[https://doi.org/10.1126/sciadv.abn2225]

-
Y. Roh, M. Kim, S.M. Won, D. Lim, I. Hong, S. Lee, T. Kim, C. Kim, D. Lee, and S. Im, “Vital signal sensing and manipulation of a microscale organ with a multifunctional soft gripper”, Sci. Robot., Vol. 6, No. 59, p. eabi6774, 2021.
[https://doi.org/10.1126/scirobotics.abi6774]

-
D. Liu, D. Zhang, Z. Sun, S. Zhou, W. Li, C. Li, W. Li, W. Tang, and Z. L. Wang, “Active-Matrix Sensing Array Assisted with Machine-Learning Approach for Lumbar Degenerative Disease Diagnosis and Postoperative Assessment”, Adv. Funct. Mater., Vol. 32, No. 21, p. 2113008, 2022.
[https://doi.org/10.1002/adfm.202113008]

-
J. Hu, Y. Qiu, X. Wang, L. Jiang, X. Lu, M. Li, Z. Wang, K. Pang, Y. Tian, and W. Zhang, “Flexible six-dimensional force sensor inspired by the tenon-and-mortise structure of ancient Chinese architecture for orthodontics”, Nano Energy, Vol. 96, p. 107073, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107073]

-
J. Qin, X. Yang, C. Shen, Y. Chang, Y. Deng, Z. Zhang, H. Liu, C. Lv, Y. Li, and C. Zhang, “Carbon nanodot-based humidity sensor for self-powered respiratory monitoring”, Nano Energy, Vol. 101, p. 107549, 2022.
[https://doi.org/10.1016/j.nanoen.2022.107549]

-
H. Ding, G. Lv, X. Cai, J. Chen, Z. Cheng, Y. Peng, G. Tang, Z. Shi, Y. Xie, and X. Fu, “An Optoelectronic thermometer based on microscale infrared-to-visible conversion devices”, Light: Sci. Appl., Vol. 11, No. 1, pp. 1–8, 2022.
[https://doi.org/10.1038/s41377-022-00825-5]

-
S. Tachibana, Y.-F. Wang, T. Sekine, Y. Takeda, J. Hong, A. Yoshida, M. Abe, R. Miura, Y. Watanabe, and D. Kumaki, “A Printed Flexible Humidity Sensor with High Sensitivity and Fast Response Using a Cellulose Nanofiber/Carbon Black Composite”, ACS Appl. Mater. Interfaces, Vol. 14, No. 4, pp. 5721–5728, 2022.
[https://doi.org/10.1021/acsami.1c20918]

-
J. Lee, S.J. Ihle, G.S. Pellegrino, H. Kim, J. Yea, C.-Y. Jeon, H.-C. Son, C. Jin, D. Eberli, and F. Schmid, “Stretchable and suturable fibre sensors for wireless monitoring of connective tissue strain”, Nat. Electron., Vol. 4, No. 4, pp. 291–301, 2021.
[https://doi.org/10.1038/s41928-021-00557-1]

-
C. Y. Kim, M. J. Ku, R. Qazi, H. J. Nam, J. W. Park, K. S. Nam, S. Oh, I. Kang, J.-H. Jang, and W. Y. Kim, “Soft subdermal implant capable of wireless battery charging and programmable controls for applications in optogenetics”, Nat. Commun., Vol. 12, No. 1, pp. 1–13, 2021.
[https://doi.org/10.1038/s41467-020-20803-y]

-
H. Ouyang, Z. Li, M. Gu, Y. Hu, L. Xu, D. Jiang, S. Cheng, Y. Zou, Y. Deng, B. Shi, W. Hua, Y. Fan, Z. Li, and Z. Wang, “A Bioresorbable Dynamic Pressure Sensor for Cardiovascular Postoperative Care”, Adv. Mater., Vol. 33, No. 39, p. 2102302, 2021.
[https://doi.org/10.1002/adma.202102302]

-
D. Lu, S. Li, Q. Yang, H.M. Arafa, Y. Xu, Y. Yan, D. Ostojich, W. Bai, H. Guo, C. Wu, S. Li, L. Jacobson, A. M. Westman, M. R. MacEwan, Y. Huang, M. Pet, and J. A. Rogers, “Implantable, wireless, self-fixing thermal sensors for continuous measurements of microvascular blood flow in flaps and organ grafts”, Biosens.Bioelectron., Vol. 206, p. 114145, 2022.
[https://doi.org/10.1016/j.bios.2022.114145]

-
J. Li, Y. Liu, L. Yuan, B. Zhang, E.S. Bishop, K. Wang, J. Tang, Y.-Q. Zheng, W. Xu, S. Niu, L. Beker, T. L. Li, G. Chen, M. Diyaolu, A.-L. Thomas, V. Mottini, J. B. H. Tok, J. C. Y. Dunn, B. Cui, S. P. Pașca, Y. Cui, A. Habtezion, X. Chen, and Z. Bao, “A tissue-like neurotransmitter sensor for the brain and gut”, Nat., Vol. 606, pp. 94–101, 2022.
[https://doi.org/10.1038/s41586-022-04615-2]
