Enhanced Moisture Resistance of Salt Core through 2D Kaolinite Colloidal Solution Coating
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study aimed to improve the moisture resistance of salt cores by investigating the suitability of a two-dimensional kaolinite colloidal solution and a commercially available SiO2 ink solution as coating agents. X-ray diffraction analysis (XRD) results showed that the intercalation of urea into kaolinite did not significantly change its layer structure. Scanning electron microscopy (SEM) images revealed that the dip-coating only affected the surface of the salt core, and the texture of the surface is differ depending on the coating solution. The humidity absorption test results showed that both coatings reduced the hygroscopicity of the salt core by more than 50%. However, in the water-solubility test, the kaolinite dissolved with the salt core, whereas the SiO2-coated salt core left a residue. These results strongly suggest that with the coating of the exfoliated kaolinite solution, salt core will remain stable in humid environments.
Keywords:
Moisture resistance, Salt core, 2D kaolinite1. INTRODUCTION
In recent years, the emerging field of electric vehicles had a great need for lightweight structures to reduce the vehicle weight and increase the mileage per charge. [1,2] In addition, the automobile industry is gradually increasing the use of aluminum alloys, which have advantages such as weight reduction, recyclability, single product manufacturing, and crash stability. Furthermore, the global automotive industry has several plans to aggressively expand the use of aluminum. [3-5] Recently, high pressure die casting and squeeze casting methods have been widely used to manufacture aluminum alloy products with high strength and excellent wear resistance. However, products with complex shapes such as automobile cylinder blocks, cylinder heads, and water pumps that are hollow or have undercuts, are manufactured using sand casting, gravity casting, or low-pressure casting methods. To assist in these processes, the use of collapsible cores, which collapse after casting and are easily removed from the product, is becoming common. The material of salt core is salt, which is a water-soluble substance. Salt core can be made of different types of salt, such as NaCl (sodium chloride) or NaCl-Na2CO3 (sodium chloride-sodium carbonate) mixture. A collapsible salt core has good moldability, does not require a binder, easily disintegrates in water, and has excellent economic feasibility. This type of environmentally friendly collapsible core is selectively used. However, salt cores produced by sintering molds are vulnerable to moisture, and will absorb moisture from the atmosphere if exposed to a humid environment for a long time before being used for casting aluminum parts. This may reduce the product value and cause defects in aluminum alloy products. To improve moisture resistance, various methods have been attempted, such as adding kaolinite during salt synthesis or mixing a glass material with a polymer material to coat the surface. [6,7,8]
In this study, to improve the moisture resistance by suppressing the moisture absorption of the salt core itself, two solutions were selected and applied to salt cores using the dip-coating method to compare the moisture resistance. The two tested solutions were a two-dimensional materialized kaolinite colloidal solution through the exfoliation process by inserting urea and a commercially available SiO2 ink solution.
2. EXPERIMENTAL
The salt core for coating was provided by Castman, Inc. (Korea). An eco-friendly and quick-drying SiO2 ink solution was purchased from Changsung Nanotech, Co. (Korea). Kaolinite, ethanol and urea were purchased from Daejung (Korea) and Sigma-Aldrich (USA). All other chemicals were reagent grade and were used without further purification unless otherwise noted
2.1 SiO2 ink solution
The SiO2 ink solution (SiO2 solution) is a transparent liquid with 15% SiO2 content and a viscosity of 5-10 cps. The SiO2 solution was used as raw material without any treatment.
2.2. Exfoliated kaolinite colloidal solution (kaolinite solution)
Kaolinite (Al2Si2O5(OH)4), as a natural clay, is a layered aluminosilicate with a 1:1 layer structure. A nanosheet solution was prepared through ion intercalation and exfoliation of kaolinite [9,10]. The raw kaolinite samples were mixed with urea ((NH2)2CO) to form intercalated kaolinite. For the kaolinite sample with the urea inserted, 3 g of kaolinite was mixed with 1.5 g of urea in a mortar and pestle for a period of time. The mixture was then stored in an oven for 48 h at 95 oC, conditions routinely used in many laboratories. The product is referred to as the kaolinite–urea intercalation. The obtained solid samples were washed three times with distilled water and absolute ethanol followed by drying at 60 oC for 24 h. For delaminating of kaolinite, the intercalated kaolinite samples were subsequently heated at 120 oC for another 1 h [11,12]. The obtained samples were mixed with ethanol and ultra-sonicated for 1 h for exfoliation. The sonicated samples were centrifuged at 1500 rpm for 10 min to obtain a coating solution by taking only the upper solution. X-ray diffraction (XRD) analysis and scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) observation were performed to determine the characteristics of the kaolinite intercalated with urea.
2.3. Coatings and measurement
The salt cores were prepared as long cylindrical samples with ∅ 10 × 30 mm3 and then coated. The coating was performed using a dip-coating method that wetted the entire area. Samples before coating were dried at 65 oC for one day in a dry oven. The coated sample were dried at 150 oC for 10 min for the SiO2 solution and at room temperature for the kaolinite solution coating. The number of coatings proceeded from 1 to 5 for the SiO2 solution and from 1 to 15 for the kaolinite solution. The fracture surface of the coating sample was investigated using SEM and the composition of the coating surface was analyzed using EDS. The moisture resistance was assessed by comparing the humidity absorption rates of the specimens; that is the higher the humidity absorption rate the lower the moisture resistance. The moisture resistance test was maintained at 30 oC and 60 % humidity for 24 h using constant temperature and humidity equipment (Laboratory Climate Chambers, WKL-64/70, WEISS), and the humidity absorption rate was calculated using the following equation:
| (1) |
where H represents the humidity absorption rate of the specimen, Wt is the mass of the specimen exposed to a constant temperature and relative humidity of 30 ºC and 60 % for 24 h, and Wo is the initial mass of the specimen.
The water solubility of the coated salt core was tested because the coating agent should not hinder the ability of the salt core to easily decompose in water. The sample was immersed in distilled water and the change over time was observed.
3. RESULTS AND DISCUSSIONS
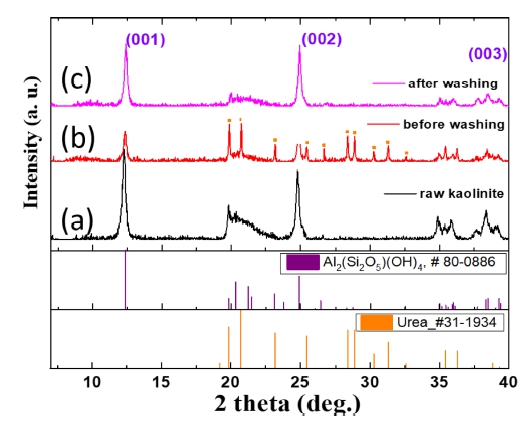
Fig. 1 shows the X-ray diffraction (XRD) patterns of the raw kaolinite, the urea-kaolinite sample before washing, and the urea-kaolinite sample after washing through the intercalation process. It is clear that the obtained sample (Fig. 1(c)) exhibits the same characteristic reflection as the raw kaolinite (Fig. 1(a)). After the intercalation of urea, the (001) reflection shifted to a lower angle owing to the expansion of the interlayer space (Fig. 1(b)).
Furthermore, the yellow dots in the diagram represent the peaks of the inserted urea, which became invisible after washing, indicating that the intercalated urea was removed. This shows that the layer structure of kaolinite did not change significantly before (Fig. 2(a)) and after (Fig. 2(b)) urea insertion, as shown in the SEM image in Fig. 2.
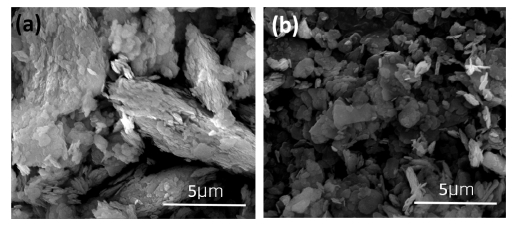
Fig. 3 illustrates the microstructure of the cross-section and surface of the salt cores that were coated with SiO2 and kaolinite solutions. Figs. 3 (a) - (c) show SEM images of the cross-section of the salt core. Table 1 shows the SEM-EDS data of Fig. 3 (a)-(c). SEM-EDS results clearly reveals that the surface and inner layers of the uncoated salt core in (a) have the same composition. However, for the salt cores coated with the SiO2 solution (b) and kaolinite solution (c), elements not included inside are found on the coating surface layer. It can be observed that the dip-coating does not affect the inside of the salt core and only affects the surface when coated with a thickness of several μm. Fig. 3(d) - (f) show the SEM images of the surface, demonstrating that the texture is different depending on the coating solution. The SiO2 solution coating shows a film formed on the surface (e). In contrast, the surface of the salt core coated with exfoliated kaolinite solution is covered with fine particles (f). These fine particles are exfoliated kaolinite particles as shown in the SEM-EDS results (Table 1).
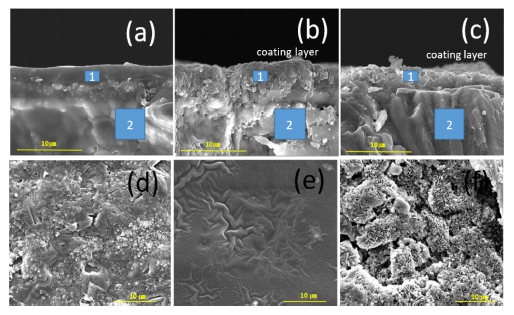
Kaolinite and SiO2 ink coating fracture surface EDS & SEM images. (a),(d) raw salt core; (b),(e) SiO2 solution; (c),(f) kaolinite solution.
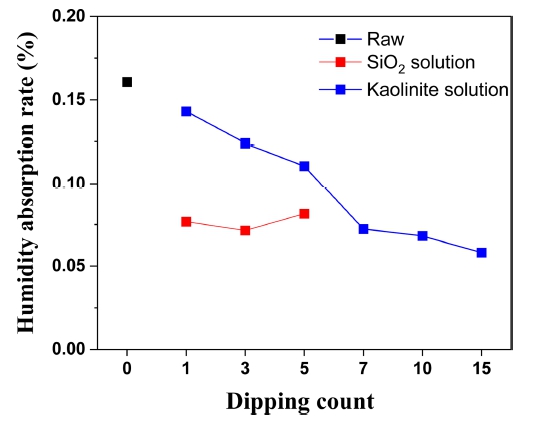
Fig. 4 shows the results of the humidity absorption test used to evaluate the humidity resistance of the coated sample. The humidity absorption results for the coating with SiO2 and kaolinite solutions are shown in Fig. 4. The non-coated salt core (black square), which showed hygroscopicity value of 0.16%, exhibited a value around 0.075% when the SiO2 solution coating (red squares) was applied, indicating that the hygroscopicity was reduced by approximately 50%. Compared to the uncoated salt cores, the salt cores coated with SiO2 solution were more resistant to humidity, regardless of the number of coatings.
Even when the number of coatings increased, the humidity absorption rate was similar to that of a single coating, indicating the effect of the SiO2 solution is not dependent on the number of coatings.
The humidity absorption of the exfoliated kaolinite-coated sample (blue squares) decreased as the number of coatings increased. Starting from the seventh coating, which exhibited a value of less than 0.075 %, the rate of the humidity absorption decreased, and the hygroscopicity values were better than those of the of SiO2 solution coating. Therefore, it can be concluded that exfoliated kaolinite coating must be applied more than seven times to stabilize the surface humidity absorption rate of the salt core.
Fig. 5. shows the dissolution results of the coated and uncoated salt cores. Both the uncoated salt cores (a, d) and kaolinite-coated salt cores (c, f) were completely dissolved after 15 h. However, the SiO2 solution-coated salt cores (b, e) did not completely dissolve in water. Instead, the SiO2 residue (dotted area) floated on top of the water. The inset in Fig. 5(e) shows the dried residue. It is believed that the resin-based organic substances in the SiO2 solution remain insoluble in water.
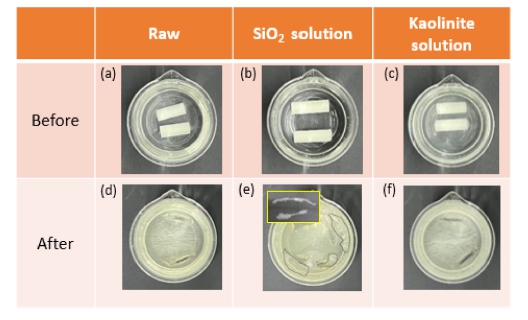
Dissolution photographs of salt cores coated with SiO2 and kaolinite solutions and uncoated salt cores.
Both coating materials are environmentally friendly solutions for castings with salt cores. However, using the kaolinite solution as a coating is more appropriate for casting with salt cores. The kaolinite solution is more suitable than the SiO2 solution because, the latter is not entirely water-soluble and requires a drying process at 150oC unlike the former, which is dried at room temperature.
4. CONCLUSIONS
This study investigated the use of exfoliated kaolinite and SiO2 solutions as coating materials for salt cores used in metal casting. The XRD and SEM analyses indicated that the layer structure of kaolinite did not change significantly after intercalation with urea. The dip-coating through only affected the surface of the salt core. Coating the salt cores with SiO2 and kaolinite solution reduced their hygroscopicity by more than 50%. Considering the solubility and preparation process, it can be concluded that kaolinite solutions are suitable for improving the moisture resistance of salt cores.
Acknowledgments
This research was supported by the Green New Deal 100 Promising Company 100 R&D Program (2MRC190) funded by the Ministry of SMEs and Startups (MSS, Korea).
REFERENCES
-
W. J. Joost, “Reducing Vehicle Weight and Improving U.S. Energy Efficiency Using Integrated Computational Materials Engineering”, JOM-J Miner. Metal M., Vol. 64, pp. 1032-1038, 2012.
[https://doi.org/10.1007/s11837-012-0424-z]

-
W. Zhang and J. Xu, “Advanced lightweight materials for Automobiles: A”, Mater. Des., Vol. 221, pp. 110994(1)-110994(20), 2022.
[https://doi.org/10.1016/j.matdes.2022.110994]

-
S. Funke and P. Plotz, “A comparison of different means to increase daily range of electric vehicles - the potential of battery sizing increased vehicle efficiency and charging infrastructure”, IEEE Vehicle Power and Propulsion Conf. (VPPC), Karlsruhe, pp. 1-6, 2014.
[https://doi.org/10.1109/VPPC.2014.7006995]

-
M. Delogua, L. Zanchia, C.A. Dattiloa, and M. Pierini, “Innovative composites and hybrid materials for electric vehicles lightweight design in a sustainability perspective”, Mater. Today Comm., Vol. 13, pp. 192-209, 2017.
[https://doi.org/10.1016/j.mtcomm.2017.09.012]

-
L. Nicoletti, A. Romano, A. König, P. Köhler, M. Heinrich, and M. Lienkamp, “An Estimation of the Lightweight Potential of Battery Electric Vehicles”, Energies, Vol. 14, No. 15, pp. 4655(1)-4655(29), 2021.
[https://doi.org/10.3390/en14154655]

-
F. Liu, Z Fan, X, Liu, Y, Huang, and P. Jiang, “Effect of Surface Coating Strengthening on Humidity Resistance of Sodium Silicate Bonded Sand Cured by Microwave Heating”, Mater. Manuf. Process, Vol. 31, pp. 1639-1642, 2016.
[https://doi.org/10.1080/10426914.2015.1117631]

-
L. Zhang, L. Zhang, and Y. Li, “Effect of kaolin on tensile strength and humidity resistance of a water-soluble potassium carbonate sand core”, Res. Dev. China Foundry, Vol. 13, No. 1, pp. 15-21, 2016.
[https://doi.org/10.1007/s41230-016-5041-y]

-
S. Tu, F. Liu, G. Li, W. Jiang, X. Liu, and Z. Fan, “Fabrication and characterization of high-strength water-soluble composite salt core for zinc alloy die castings”, Int. J. Adv. Manuf. Technol., Vol. 95, pp. 505-512, 2018.
[https://doi.org/10.1007/s00170-017-1208-y]

-
A. Shawky, S. M. El-Sheikh, M. N. Rashed, S. M. Abdo, and T. I. El-Dosoqy, “Exfoliated kaolinite nanolayers as an alternative photocatalyst with super activity”, J. Environ. Chem. Eng., Vol. 7, p. 103174, 2019.
[https://doi.org/10.1016/j.jece.2019.103174]

-
T. Kristóf, Z. Sarkadi, Z. Ható, and G. Rutkai, “Simulation study of intercalation complexes of kaolinite with simple amides as primary intercalation reagents”, Comput. Mater. Sci., Vol. 143, pp. 118-125, 2018.
[https://doi.org/10.1016/j.commatsci.2017.11.010]

-
M. N. Niu and C. X. Guo, “Preparation of delaminated nano-kaolinite by intercalation of chemical assistants”, Adv. Mater. Res., Vol. 11-12, pp. 441-444, 2006.
[https://doi.org/10.4028/www.scientific.net/AMR.11-12.441]

-
M. Valášková, M. Rieder, V. Matějka, P. Čapková, and A. Slíva, “Exfoliation/delamination of kaolinite by low-temperature washing of kaolinite–urea intercalates”, Appl. Clay Sci., Vol. 35, pp. 108-118, 2007.
[https://doi.org/10.1016/j.clay.2006.07.001]