Development of a MEMS-based H2S Sensor with a High Detection Performance and Fast Response Time
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
H2S is a toxic and harmful gas, even at concentrations as low as hundreds of parts per million; thus, developing an H2S sensor with excellent performance in terms of high response, good selectivity, and fast response time is important. In this study, an H2S sensor with a high response and fast response time, consisting of a sensing material (SnO2), an electrode, a temperature sensor, and a micro-heater, was developed using micro-electro-mechanical system technology. The developed H2S sensor with a micro-heater (circular type) has excellent H2S detection performance at low H2S concentrations (0–10 ppm), with quick response time (<16 s) and recovery time (<65 s). Therefore, we expect that the developed H2S sensor will be considered a promising candidate for protecting workers and the general population and for responding to tightened regulations.
Keywords:
Gas sensors, Oxide semiconductors, SnO2, Hydrogen sulfide, MEMS1. INTRODUCTION
The quick and safe detection of hydrogen sulfide (H2S) gas, which is a toxic, harmful, corrosive, and colorless gas, is an essential concern for the health and safety of industrial workers and the general population. H2S gas with a rotten-egg smell is generated from natural sources, such as petroleum, natural gas, and volcanic gas, and human sources, such as petroleum and natural gas extraction and purification, paper and pulp manufacturing, textile production, chemical manufacturing, and wastewater treatment [1-5]. According to the Occupational Safety and Health Administration (OSHA), H2S is considered a dangerous gas comparable to carbon monoxide (CO) in the workplace. Danger to H2S exposure is determined by its concentration and is categorized into three groups: (1) acute exposure (>300 ppm), which causes collapsing, unconsciousness, and death; (2) post-acute exposure (>100 ppm) over 30 min, which causes difficulty in breathing and comas; (3) chronic exposure (<1 ppm) over several days, which causes nausea, headache, and skin/eye irritation.
Regulations for workers and the general population susceptible to H2S have been developed and tightened; thus, the development of an H2S sensor with excellent performance, such as high response and good selectivity, is becoming increasingly important. Various methods for detecting H2S are available, including semiconducting metal oxide, electrochemical, and optical methods. Among these methods, H2S sensors utilizing semiconducting metal oxides (SnO2, ZnO, WO3, and CuO) as sensing materials have been steadily developed over a long time [6-16]. Despite the advantages of semiconducting metal oxides for H2S detection, their H2S detection performance must be improved by tightening regulations for workers and the general population susceptible to H2S [17-24].
In this study, a H2S sensor with a high and fast response was developed using tin dioxide (SnO2) and an optimized micro-heater to respond to tightened regulations. SnO2 is the most widely used sensing material in semiconducting metal oxide-based gas sensors because of its excellent gas detection ability (a good compromise between price, stability, and reliability of the material, fast response, and recovery time) and many advantages for fabrication (low-cost, simple fabrication, and good compatibility with micro-electro-mechanical system (MEMS) processes) [9, 14].
A SnO2-based sensor detects H2S through a resistance-change mechanism, which is primarily an induced variation of depletion region owing to the adsorption of ionized oxygen species (O2−, O−, and O2−) on the SnO2 surface. The oxygen-related gas-sensing mechanism involves the absorption of oxygen molecules on the SnO2 surface to generate chemisorbed oxygen species (O2−, O−, and O2−) by capturing electrons from the conductance band, which causes the SnO2 surface to be highly resistive. SnO2 is exposed to traces of reductive gases. When reacting with the oxygen species in SnO2, the reductive gas reduces the concentration of oxygen species on the surface, thereby increasing the electron concentration.
In this study, a gas sensor for detecting H2S, consisting of a micro-heater, sensing material, and electrode, was fabricated using MEMS technology. To improve the H2S detection ability governed by the principle mentioned above, we embedded a micro-heater in the H2S sensor to increase the temperature. The micro-heater offers proper thermal energy for the reaction between the target gas (H2S) and the sensing material (SnO2). Thus, the performance of the gas sensor can be dramatically improved. To satisfy these requirements, we designed and characterized an MEMS-based H2S sensor with micro-heaters (line and circular types). Microheaters with different designs were fabricated on the proposed H2S sensor platform and characterized To investigate the relationship between the H2S detection performance and heating performance influenced by the design of the micro-heater. Finally, H2S was detected using an optimized micro-heater installed on the fabricated H2S sensor.
2. DESIGN AND FABRICATION
The MEMS-based H2S sensor with micro-heaters (line and circular types) was designed as shown in Fig. 1. The proposed MEMS-based H2S sensor consisted of line and circular micro-heaters, a temperature sensor, an interdigitated electrode (IDE), and a sensing material (SnO2). In particular, the different types of micro-heaters improve the performance of the MEMS-based H2S sensor compared with previous types (meander, rectangular, and rectangular mesh types) because the continual improvement of the H2S detection ability of the sensor by considering the dangers of H2S gas is important. The sizes of the entire sensor and sensing area were 3 mm ⅹ 3 mm and 100 μm ⅹ100 μm, respectively. The width and thickness of two types of micro-heaters, temperature sensor and IDE were 20 μm and 200 nm, respectively. To minimize the loss of thermal energy generated by the micro-heater, we used a quartz wafer was used as the sensor substrate, which can also minimize the fabrication cost and difficulty level. Pt, which has a linear relationship between temperature and resistance, was used to fabricate the micro-heater and temperature sensor. Au and tin dioxide (SnO2) were used as the IDE and sensing materials, respectively. Fig. 2(a) and (b) show the fabrication process and a photograph of the proposed MEMS-based H2S, respectively.
The MEMS-based H2S sensor was fabricated as follows. First, the quartz wafer used as the substrate was cleaned with acetone and methanol solution for 10 min. Subsequently, the proposed micro-heaters (line and circular) and temperature sensors were fabricated through a photolithography process to pattern the desired design and an e-beam evaporation process for Pt deposition. Silicon nitride (Si3N4), which was used as an electrical insulating and passivation layer, was deposited using plasma-enhanced chemical vapor deposition (PECVD). The IDE was fabricated using photolithography and e-beam evaporation processes for Au deposition. Finally, SnO2, as the H2S sensing material, was deposited via sputtering, and Si3N4 was etched to fabricate the electrical pads of the micro-heaters and temperature sensor.
3. RESULTS AND DISCUSSIONS
The performance of the fabricated temperature sensor and line and circular micro-heaters was characterized before estimating the H2S detection ability of the proposed MEMS-based H2S sensor. First, the resistance of the fabricated temperature sensor and the temperature change were measured using a commercial ceramic heater installed in the gas chamber.
The measured resistance of the temperature sensor increased linearly, as shown by the blue line in Fig. 3. Thus, the heating performance of the line and circular micro-heaters could be estimated from the measured resistance of the temperature sensor in real time. Different input voltage values were applied to the line and circular micro-heaters, and their heating performances were characterized by measuring the resistance of the temperature sensor, as shown by the red line in Fig. 3.

Measured resistance of the temperature sensor as functions of the controlled temperature and input voltage of the micro-heater.
The excellent H2S detection ability (good response and selectivity, fast response and recovery times) of SnO2 is ensured at operating temperature in the range 120–200 oC. Therefore, an input voltage was applied to the micro-heater to increase the optimum H2S detection temperature of SnO2. By measuring resistance of temperature sensor, the expected temperatures of SnO2 were approximately 120, 145, and 165 oC when input voltages in the range of 3–4 V were applied to the circular micro-heater and approximately 110, 140, and 160 oC when input voltages in the range of 5–7 V are applied to line micro-heater. This implied that the circular micro-heater produced more thermal energy than the line micro-heater. Increasing the current through the micro-heater is important because its heating performance is affected by Joule heating, which is closely related to the current traveling through the micro-heater (Joule heating [cal]=I2Rt). The measured resistance values of the line and circular micro-heaters were 87.37 and 30.27 Ω, respectively. The circular micro-heater had a superior heating performance to a line micro-heater because more current flowed when an equal or lower input voltage was applied to it. Thermal energy in the line and circular micro-heaters was generated nonlinearly along with the applied input voltage, and the circular micro-heater exhibited a stronger nonlinearity, as shown in Fig. 3. This was because the generated thermal energy was proportional to the square of the current flowing through the micro-heater (Joule heating [cal]=I2Rt). Based on these experimental results, we expect that the circular micro-heater can supply proper thermal energy; thus, the MEMS-based H2S sensor with the circular micro-heater can detect H2S effectively.
Next, the H2S detection ability of the MEMS-based H2S sensor with the line and circular micro-heaters was characterized. The fabricated MEMS-based H2S sensor was placed in the prepared chamber, as shown in Fig. 4, and H2S gas in the range of 0–10 ppm was injected into the chamber by measuring the output currents of the MEMS-based H2S sensor. The output current of the MEMS-based H2S sensor increased when H2S was injected into the chamber, as shown in Fig. 5(a). This was because the oxygen species adsorbed on the sensing material surface (SnO2) was consumed by the chemical reaction, and electrons were donated back to the SnO2 surface, resulting in a decrease in the electrical resistance when the MEMS-based H2S sensor was exposed to H2S. Therefore, the measured output current of the MEMS-based H2S sensor increased. The response of a MEMS-based H2S sensor is frequently defined as

(a) Measured output current of fabricated MEMS-based H2S with line and circular micro-and H2S concentration; (b) measured response/recovery times of fabricated MEMS-based H2S with line and circular micro-heaters.
where Rair and Rgas are the resistances, and Iair and Igas are the conductances of the sensor in air and the reducing gas (H2S), respectively.
The response time is defined as the time required to decrease the resistance or increase the conductance of the H2S sensor by 90% of the total decrease (Rair-Rgas) or total increase (Igas-Iair). The recovery time is defined as the time required to recover the resistance or increase the conductance of the H2S sensor by 90% of the total decrease (Rair-Rgas) or total increase (Igas-Iair) when H2S injection is stopped and air is injected into the chamber. Fig. 5(a) shows the measured responses of the fabricated MEMS-based H2S sensor at different H2S concentrations (0–10 ppm), and Fig. 5(b) shows the measured response/recovery time of the fabricated MEMS-based H2S sensor.
The response of the sensor to H2S dramatically improved when the operating temperature was increased by increasing the input voltage of the line and circular micro-heaters. The response values of the MEMS-based H2S sensor with the line micro-heater (Igas/Iair) for H2S concentration of 0–10 ppm were 5.03, 5.89, 6.25, 6.84, 7.43 at an input voltage of 7 V; 4.37, 6.28, 7.30, 8.31, 8.41 at 6 V; and 4.75, 6.46, 7.51, 8.54, 9.40 at 5 V. With the circular micro-heater, the response values (Igas/Iair) for a H2S concentration 0–10 ppm were 4.75, 6.15, 6.99, 7.73, 8.25 at an input voltage 4 V; 4.01, 5.26, 6.09, 6.65, 7.06 at 3.5 V ; and 4.22, 6.16, 7.56, 9.02, 9.16 at 3 V . As mentioned earlier, the both MEMS-based H2S sensors with line and circular micro-heaters can detect H2S gas in temperature range 120–200 oC. In particular, the fabricated MEMS-based H2S sensor with a circular micro-heater has superior H2S detection ability at a lower micro-heater input voltage compared with the sensor with a line micro-heater. This is because the circular micro-heater has a remarkable heating performance, as shown in Fig. 3. The response and recovery times of the MEMS-based H2S sensor were reduced by more than twofold when the circular micro-heater was utilized. These results demonstrate that the proposed and fabricated MEMS-based H2S sensors can detect H2S gas more quickly and accurately.
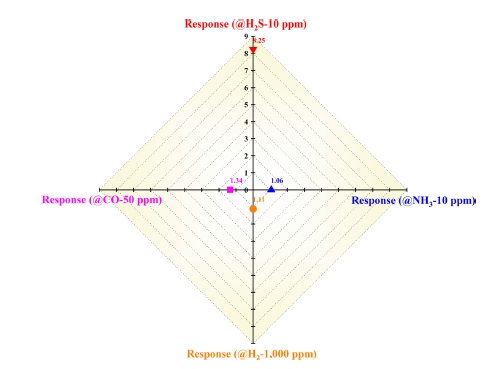
Finally, the fabricated MEMS-based H2S sensor with the circular micro-heater not only has a strong response but also good selectivity toward the target gas (H2S in this study) for practical applications. To estimate the selectivity of the MEMS-based H2S sensor with a circular micro-heater, the sensor was exposed to different gases, including ammonia (NH3), hydrogen (H2), and carbon monoxide (CO) because they react well with SnO2 under various conditions. Generally, these gases react well with SnO2 at different temperatures and H2S reacts well with SnO2 between 120 and 200 oC. As shown in Fig. 6, the fabricated MEMS-based H2S sensor with the circular micro-heater had a high selectivity toward H2S. This was because the fabricated MEMS-based H2S sensor was effective under temperature conditions (120–200 oC) in which H2S reacts well with SnO2. If the sensor operated outside the optimum temperature range, its selectivity toward H2S would have been very low. Based on the experimental results, we confirmed the importance of supplying proper thermal energy for the reaction between H2S and oxygen species (O2−, O−, and O2−) on the SnO2 surface via a micro-heater with superior heating performance. For a reaction to occur between molecules, they must exist as close to each other as possible, and each molecule must have an energy greater than the energy required for the reaction (activation energy, Ea). According to the Maxwell–Boltzmann distribution, as the temperature increases, the number of molecules with energies above the activation energy increases:
where k is Boltzmann’s constant, E is the energy, and T is the absolute temperature.
In summary, the H2S detection ability of the proposed MEMS-based H2S sensor was significantly improved by supplying thermal energy through micro-heaters embedded in the H2S sensor, which can be achieved by fabricating a well-made micro-heater with an optimized design.
4. CONCLUSIONS
In this study, a MEMS-based H2S sensor with a micro-heater was fabricated. The sensor uses a semiconducting metal oxide (SnO2) as the sensing material and consists of a substrate, sensing material, IDE, and micro-heater. As micro-heaters embedded in the proposed sensor increase the temperature, they have an important role to play because the reaction between H2S and oxygen species (O2−, O−, and O2−) on the SnO2 surface is affected by the operating temperature of the sensor. The development of a micro-heater that produces more thermal energy by minimizing power consumption or operating voltage is essential for real-time monitoring applications. To satisfy this requirement, we developed line and circular micro-heaters and characterized their heating performance by estimating the H2S detection ability of the fabricated sensor. This was accomplished by applying an input voltage and measuring the resistance of the temperature sensor. Based on the experimental results, we confirmed that the circular micro-heater produces thermal energy more effectively. Therefore, the H2S sensor fabricated with the circular micro-heater exhibited superior H2S detection ability. Its responses (Igas/Iair) were 4.75 (2 ppm), 6.15 (4 ppm), 6.99 (6 ppm), 7.73 (8 ppm), and 8.25 (10 ppm) at an applied input voltage of 4 V. Furthermore, it had a shorter response time (<16 s) and recovery time (<65 s) than the H2S sensors with a line micro-heater. H2S, which is a toxic and harmful gas, even at concentrations as low as hundreds of parts per million, is primarily produced in various fields, such as oil deposits, biogas, and natural gas. Developing an H2S sensor with good responsivity and a fast response time is crucial for the health and safety of industrial workers and the general population. Therefore, we expect that the developed and optimized H2S sensor in this study will be a good candidate for practical real-time applications.
Acknowledgments
This work was supported by the Technology Development Program (S3177927), funded by the Ministry of SMEs Startups (MSS), Korea.
This study has been conducted with the support of the Korea Institute of Industrial Technology as “Technical support project for research and development of manufacture to improve core components of eco-mobility” (Kitech JF-23-0007).
This work was supported by the Korea Innovation Foundation(INNOPOLIS) grant funded by the Korea government(MSIT) (2020-DD-UP-0348).
REFERENCES
-
S. Ghaderahmadi, M. Kamkar, N. Tasnim, M. Arjmand, and M. Hoorfar, “A review of low-temperature H2S gas sensor: fabrication and mechanism”, New J. Chem., Vol. 45, No. 38, pp. 17727-17752, 2021.
[https://doi.org/10.1039/D1NJ02468J]

-
W. Mickelson, A. Sussman, and A. Zettl, “Low-power, fast, selective nanoparticle-based hydrogen sulfide gas sensor”, Appl. Phys. Lett., Vol. 100, No. 17, pp. 173110-173113, 2012.
[https://doi.org/10.1063/1.3703761]

-
L. Mei, Y. Chen, and J. Ma, “Gas sensing of SnO2 Nanocrystal Revised: Developing Ultra-Sensitive Sensors for Detecting the H2S Leakage of Biogas”, Sci. Rep., Vol. 4, No. 1, pp. 6028-6035, 2014.
[https://doi.org/10.1038/srep06028]

-
C. Duc, M. L. Boukhenane, J. L. Wojkiewicz, and N. Redon, “Hydrogen Sulfide Detection by Sensors Based on Conductive Polymers: A Review”, Front. Mater., Vol. 7, No. 215, pp. 1-10, 2020.
[https://doi.org/10.3389/fmats.2020.00215]

-
V. Balasubramani, A. N. Ahamed, S. Chandraleka, K. K. Kumar, M. R. Kuppusamy, and T. M. Sridhar, “Highly Sensitive and Selective H2S Gas Sensor Fabricated with β-Ga2O3/rGO”, ECS J. Solid State Sci. Technol., Vol. 9, No. 5, pp. 055009-0550014, 2020.
[https://doi.org/10.1149/2162-8777/ab9a18]

-
K. Yao, D. Caruntu, Z. Zeng. M., J. Chen, C. J. O’Connor, and W. Zhou, “Parts per Billion-Level H2S Detection at Room Temperature Based on Self-Assembled In2O3 Nanoparticles”, J. Phys. Chem. C, Vol. 113, No. 33, pp. 14812-14817, 2009.
[https://doi.org/10.1021/jp905189f]

-
S. C. Zhang, Y. W. Huang, Z. Kuang, S. Y. Wang, W. L. Song, D. Y. Ao, W. Liu, and Z. J. Li, “Solvothermal Synthesized In2O3 Nanoparticles for ppb Level H2S Detection”, Nanosci. Nanotechnol. Lett., Vol. 7, No. 6, pp. 455-461, 2015.
[https://doi.org/10.1166/nnl.2015.1993]

-
J. Kim, and K. Yong, “Mechanism Study of ZnO Nanorod-Bundle Sensors for H2S Gas Sensing”, J. Phys. Chem. C., Vol. 115, No. 15, pp. 7218-7224, 2011.
[https://doi.org/10.1021/jp110129f]

-
S. M., Zhang, P. P. Zhang, Y. Wang, Y. Y. Ma, J. Zhong, and X. H. Sun, “Facile Fabrication of Well-Ordered Porous Cu-Doped SnO2 Thin Film for H2S Sensing”, ACS Appl. Mater. Interfaces, Vol. 6, No. 17, pp. 14975-14980, 2014.
[https://doi.org/10.1021/am502671s]

-
Y. B. Shen, B. Q. Zhang, X. M. Cao, D. Z. Wei, J. W. Ma, L. J. Jia, S. L. Gao, B. Y. Cui, and Y. C. Jin, “Microstructure and Enhanced H2S Sensing Properties of Pt-Loaded WO3 Thin Films”, Sens. Actuators B. Chem., Vol. 193, pp. 273-279, 2014.
[https://doi.org/10.1016/j.snb.2013.11.106]

-
Y. W. Huang, W. M. Chen, S. C. Zhang, Z. Kuang, D. Y. Ao, N. R. Alkurd, W. L. Zhou, W. Liu, W. Z. Shen, and Z. J. Li, “A High Performance Hydrogen Sulfide Gas Sensor Based on Porous α-Fe2O3 Operates at Room-Temperature”, Appl. Surf. Sci., Vol. 351, pp. 1025-1033, 2015.
[https://doi.org/10.1016/j.apsusc.2015.06.053]

-
Z. Li, Y. Huang, S. Zhang, W. Chen, Z. Kuang, D. Ao, W. Liu, and Y. Fu, “A Fast Response & Recovery H2S Gas Sensor Based on α-Fe2O3 Nanoparticles with ppb Level Detection Limit”, J. Hazard. Mater., Vol. 300, pp. 167-174, 2015.
[https://doi.org/10.1016/j.jhazmat.2015.07.003]

-
S. Steinhauer, E. Brunet, T. Maier, G. C. Mutinati, A. Köck, O. Freudenberg, C. Gspan, W. Grogger, A. Neuhold, and R. Resel, “Gas Sensing Properties of Novel CuO Nanowire Devices”, Sens. Actuators B. Chem., Vol. 187, pp. 50-57, 2013.
[https://doi.org/10.1016/j.snb.2012.09.034]

-
S. W. Choi, A. Katoch, J. Zhang, and S. S. Kim, “Electrospun Nanofibers of CuO−SnO2 Nanocomposite as Semiconductor Gas Sensors for H2S Detection”, Sens. Actuators B. Chem., Vol. 176, pp. 585-591, 2013.
[https://doi.org/10.1016/j.snb.2012.09.035]

-
X. Li, Y. Wang, Y. Lei, and Z. Gu, “Highly Sensitive H2S Sensor Based on Template Synthesized CuO Nanowires”, RSC Adv., Vol. 2, No.2, pp. 2302-2307, 2012.
[https://doi.org/10.1039/c2ra00718e]

-
F. Zhang, A. Zhu, Y. Luo, Y. Tian, J. H. Yang, and Y. Qin, “CuO Nanosheets for Sensitive and Selective Determination of H2S with High Recovery Ability”, J. Phys. Chem. C, Vol. 114, No. 45, pp. 19214-19219, 2010.
[https://doi.org/10.1021/jp106098z]

-
T. N. H. Tran, D. H. Nguyen, V. D. Nguyen, M. H. Chu, T. T. L. Dang, V. T. Nguyen, H. P. Nguyen, and V. H. Nguyen, “An effective H2S sensor based on SnO2 nanowires decorated with NiO nanoparticles by electron beam evaporation”, RSC Adv., Vol. 9, No. 24, pp. 13887-13895, 2019.
[https://doi.org/10.1039/C9RA01105F]

-
Q. Zhou, L. Xu, A. Umar, W. Chen, and R. Kumar, “Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications”, Sens. Actuators B. Chem., Vol. 256, pp. 656-664, 2018.
[https://doi.org/10.1016/j.snb.2017.09.206]

-
P. M. Bulemo, H. J. Cho, D. H. Kim, and I. D. Kim, “Facile Synthesis of Pt-Functionalized Meso/Macroporous SnO2 Hollow Spheres through in Situ Templating with SiO2 for H2S Sensors”, ACS Appl. Mater. Interfaces., Vol. 10, No. 21, pp. 18183-18191, 2018.
[https://doi.org/10.1021/acsami.8b00901]

-
I. S. Hwang, J. K. Choi, S. J. Kim, K. Y. Dong, J. H. Kwon, B. K. Ju, and J. H. Lee, “Enhanced H2S sensing characteristics of SnO2 nanowires functionalized with CuO”, Sens. Actuators B. Chem., Vol. 142, No. 1, pp. 105-110, 2009.
[https://doi.org/10.1016/j.snb.2009.07.052]

-
Z. Song, J. Liu, Q. Liu, H. Yu, W. Zhang, Y. Wang, Z. Huang, J. Zang, and H. Liu, “Enhanced H2S gas sensing properies based on SnO2 quantum wire/reduced graphene oxide nanocomposites: Equilibrium and kinetics modeling”, Sens. Actuators B. Chem., Vol. 249, pp. 632-638, 2017.
[https://doi.org/10.1016/j.snb.2017.04.023]

-
Q. Zhou, W. Chen, L. Xu, R. Kumar, Y. Gui, Z. Zhao, C. Tang, and S. Zhu, “Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials”, Ceram. Int., Vol. 44, No. 4, pp. 4392-4399, 2018.
[https://doi.org/10.1016/j.ceramint.2017.12.038]

-
T. Itoh, T. Nakashima. T. Akamatsu, N. Izu, and W. Shin, “Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensor”, Sens. Actuators B. Chem., Vol. 187, pp. 135-141, 2013.
[https://doi.org/10.1016/j.snb.2012.09.097]

-
V. T. Pham, D. H. Nguyen, V. D. Nguyen, T. T. L. Dang , and V. H. Nguyen, “Enhancement of gas-sensing characteristics of hydrothermally synthesized WO3 nanorods by surface decoration with Pd nanoparticles”, Sens. Actuators B. Chem., Vol. 223, pp. 453-460, 2016.
[https://doi.org/10.1016/j.snb.2015.09.108]