Analysis Method of Volatile Sulfur Compounds Utilizing Separation Column and Metal Oxide Semiconductor Gas Sensor
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Gas chromatography (GC) separation technology and metal oxide semiconductor (MOS) gas sensors have been integrated for the effective analysis of volatile sulfur compounds (VSCs) such as H2S, CH3SH, (CH3)2S, and (CH3)2S2. The separation and detection characteristics of the GC/MOS system using diluted standard gases were investigated for the qualitative and quantitative analysis of VSCs. The typical concentrations of the standard gases were 0.1, 0.5, 1.0, 5.0, and 10.0 ppm. The GC/MOS system successfully separated H2S, CH3SH, (CH3)2S, and (CH3)2S2 using a celite-filled column. The reproducibility of the retention time measurements was at a 3% relative standard deviation level, and the correlation coefficient (R2) for the VSC concentration was greater than 0.99. In addition, the chromatograms of single and mixed gases were almost identical.
Keywords:
Gas sensor, Volatile sulfur compounds (VSCs), Metal oxide semiconductor (MOS), GC/MOS system, Column separation1. INTRODUCTION
Volatile sulfur compounds (VSCs) are generated from various anthropogenic sources, including livestock operations, chemical/textile/leather/coating manufacturing facilities, and waste/wastewater/sewage treatment facilities. Representative VSCs, including hydrogen sulfide (H2S), methyl mercaptan (CH3SH), dimethyl sulfide ((CH3)2S/DMS), and dimethyl disulfide ((CH3)2S2/DMDS), are managed as designated odorous substances under the Malodor Prevention Act of Environment (emission allowance: ≤60 ppb/H2S, ≤4 ppb/CH3SH, ≤50 ppb/DMS, ≤30 ppb/DMDS). To measure the concentration of these sulfur compounds, an instrumental analysis method, such as gas chromatography (GC), of a low-temperature concentrated sample is generally utilized. However, complex processes such as the low-temperature concentration of the odorous sample in the gaseous state and heating of the concentrated sample with a thermal desorption device are required, as demonstrated by Hong et al. [1].
To determine sulfur-containing odorants in air, a method involving a GC equipped with a flame photometric detector (FPD) with selective and high-sensitivity analysis capabilities is typically used, as in the work of Kim et al. and Seo et al. [2,3]. However, pre-processing is required to enrich the samples. Only sample concentrations as high as a few ppm can be analyzed by direct injection into a GC injector or a quantitative loop module. This makes it challenging to analyze samples taken in the field [2-4].
Various studies have been conducted on semiconductor gas sensors that can measure and monitor odors that migrate and diffuse in space and time in real time to address the difficulties in on-site analysis of sulfur-compound odor components and provide a rapid response to odor complaints. The need for this technology is growing. The minimum detection limit of the H2S gas sensor evaluated in the study by Han et al. was approximately 22 ppb, and the precision error was within approximately 1%, indicating excellent utilization for on-site odor measurement [5].
Choi et al. [6] studied a gas chromatography/metal oxide semiconductor (GC/MOS system) analyzer equipped with ultrasensitive semiconductor gas sensors for the qualitative and quantitative analysis of various odor components and found that the results were similar to trimethylamine (CH3)3N (TMA) concentration analysis by spectrophotometry, a conventional method for measuring TMA content. Hanada et al. developed a new type of simple gas analyzer by combining a highly sensitive In2O3 gas sensor with GC separation technology to measure the VSC component of halitosis, which demonstrated a VSC measurement range of 50 to 1,000 ppbv [7].
The above studies highlight that it is easier and faster to measure the concentration of odorants in air than traditional odor analysis methods, suggesting that it can be used for a variety of applications. Recently, Kim and Lee conducted basic research on the GC/MOS system and showed that it can quantitatively and qualitatively analyze approximately 16 of 22 nationally designated odorous gas substances in a single step and separate H2S, CH3SH, DMS, and DMDS [8].
In this study, we assembled a GC/MOS system combined with GC separation technology using an off-the-shelf MOS gas sensor as the detector and investigated whether individual VSC components, such as H2S, CH3SH, DMS, and DMDS, could be separated and quantified from the chromatogram results of the MOS gas sensor mounted on the back end of the column. Furthermore, the retention times (RTs) at which each VSC component was separated and maximally detected were determined. We prepared standard gas concentrations of diluted VSCs at 0.1, 0.5, 1.0, 5.0, and 10.0 ppm to calculate and measure the repeatability and output curve equations of the MOS gas sensor and compared the chromatograms of individual VSC components with those of mixed VSC components to confirm the separation characteristics of the GC/MOS system.
2. EXPERIMENTAL
2.1 Configuration of GC/MOS system
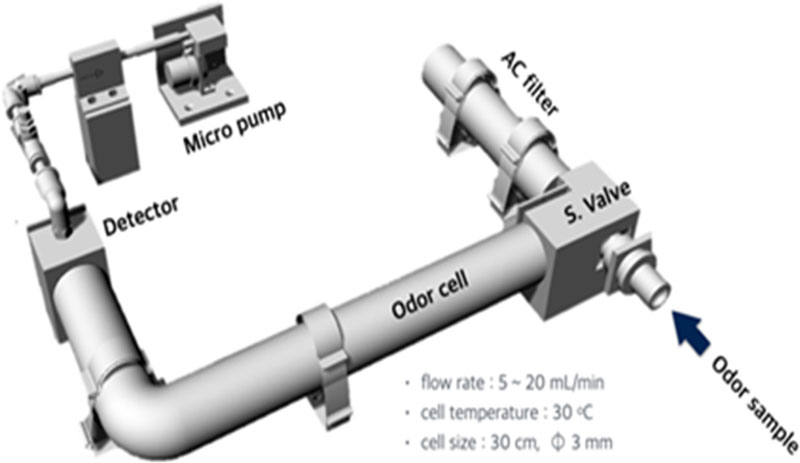
The GC/MOS system consisted of an activated carbon filter (AC filter), sample inlet (S. valve), column (odor cell), detector (MOS gas sensor), and vacuum pump (micropump), as reported by Kim et al. [9]. A small vacuum pump mounted at the back of the system sucked air from the atmosphere, which passed through an activated carbon filter to create odorless air and was fed at a constant rate to the carrier gas. Because the vacuum pump sucks air from the atmosphere, there is no need for a separate carrier gas supply, which has the advantage of miniaturizing the system. This system can also supply air containing oxygen as a carrier gas rather than an inert gas, which has a significant impact on the reaction stability and recovery characteristics of MOS gas sensors. This differs from the work of Oh, Staples, and Viswanathan, who utilized surface acoustic wave (SAW) sensors as detectors, which used the inert gas helium (He) as the carrier gas, and configured a different carrier gas supply system [10,11].
An internal schematic of the GC/MOS system is shown in Fig. 1. The column portion separating the VSC components was filled with celite in a Teflon tube, as reported by Hanada et al. [7,9]. The length and inner diameter of the celite-filled column were 30 cm and 3 mm, respectively. The celite-filled Teflon tube and temperature sensor were inserted into a copper tube with an inner diameter of 12 mm and of the same length as the Teflon tube to create a double tube. In addition, the Teflon tubes to be filled with celite were cleaned thoroughly, washed evenly with 10 N H3PO4 aqueous solution or acetone mixture (0.05 N H3PO4 + acetone), and dried thoroughly with flowing nitrogen gas to improve the reproducibility of the VSC analysis and reduce the tail drag of the VSC component peaks. The celite in the Teflon tube was filled using a vacuum pump, and the quartz glass fibers sealing the holes on both sides of the tube were treated with an acid wash [12].
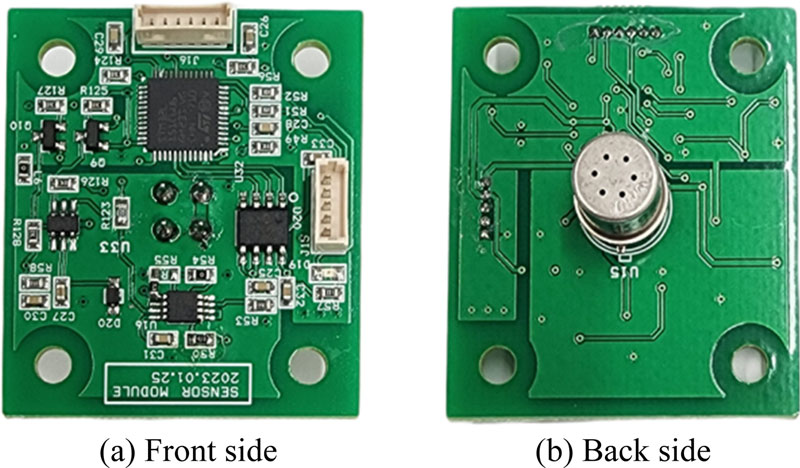
The detector mounted at the bottom of the column utilized an MOS gas sensor, which is similar to the human olfactory mechanism and responds to a variety of odorous substances. To drive the MOS gas sensor (TGS2602, Figaro Engineering Ltd., Japan) and the voltage divider circuit, 5 V DC was applied to the sensor's electrodes and heater, respectively. The voltage applied to the heater ensured that the surface temperature of the MOS gas sensor was always maintained between 400 and 450oC. In addition, the MOS gas sensor was integrated into the onboard PCB (Fig. 2), and only the gas contact part of the MOS gas sensor was inserted into the acetal manifold to fix it; it was mounted as close as possible to the end of the column so that the VSC part separated by the column part could react quickly with the MOS gas sensor.
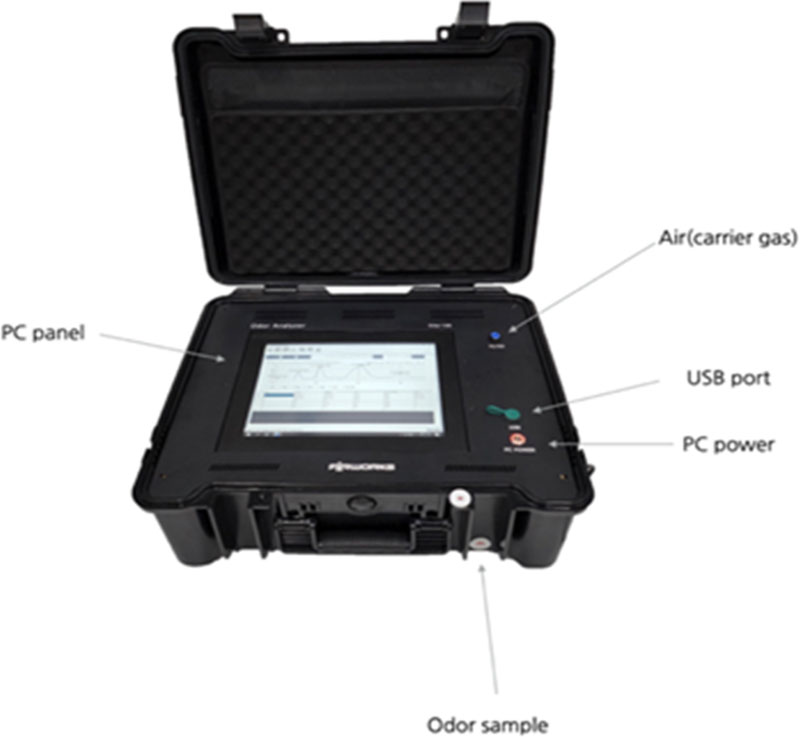
The MOS gas sensor, heater temperature, sample injection unit, and real-time measurement output were controlled by a Windows-based program. Fig. 3 shows the internal components of the GC/MOS system and PC, which were built as a single unit and made of plastic for easy transportation, as no additional accessories such as a carrier gas supply were required.
2.2 Experimental Methods
To perform quantitative and qualitative analyses of the individual odor components of the VSCs, such as H2S, CH3SH, DMS, and DMDS, a GC/MOS system with an MOS gas sensor as a detector was used, and approximately 100 ppm of each standard gas (RiGAS, Korea) in nitrogen was used. The VSC standard gas prepared in an odorless bag or a Tedlar bag was connected to the odor sample injection holder shown in Fig. 1, and approximately 4 mL of the standard gas sample was automatically injected into the column using the measurement start algorithm of the GC/MOS system. The injected standard gas sample was separated by RT, and digital signals were obtained using a chromatogram. After the standard gas sample was injected into the column, the valve at the sample inlet was automatically changed to continuously supply odorless air that passed through an activated carbon filter. The flow rate of the air-carrier gas was set to approximately 6 mL/min. In addition, the temperature of the column section formed in the space between the Teflon tube and the copper tube was set to an isothermal condition, maintained at 30oC by running a space heater installed outside the copper tube. Since components such as H2S and CH3SH are not well separated under high temperature conditions, as in the study by Kim and Lee [8], the optimal temperature condition for the column section was a relatively low 30oC isothermal condition.
Additional standard gas samples were prepared at concentrations of 0.1, 0.5, 1.0, 5.0, and 10.0 ppm by diluting 100 ppm of VSC standard gas with odorless air in a 3 L odorless bag. H2S, CH3SH, DMS, and DMDS standards prepared at 0.1, 0.5, 1.0, 5.0, and 10.0 ppm, respectively, were measured in five replicates, and the peak maxima of the components separated by the GC/MOS system were used as the concentration output (peak height). The time was used as the RT of each component. From the chromatogram results of the GC/MOS system, the maximum output values of the component peaks allowed quantitative analysis of the VSC components, and the column RT, the time at which the maximum output value was identified, allowed qualitative analysis. The detailed analysis conditions are listed in Table 1.
3. RESULTS AND DISCUSSIONS
3.1 Separation Characteristics of VSC Components
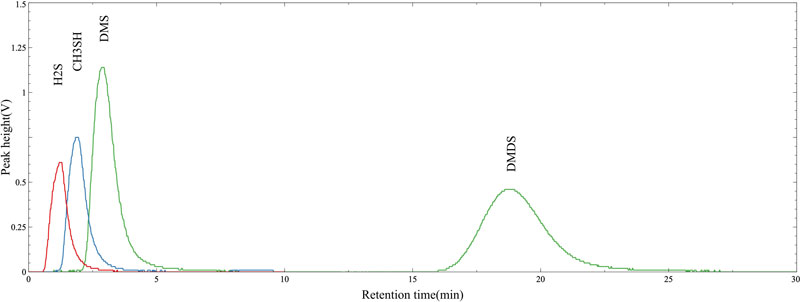
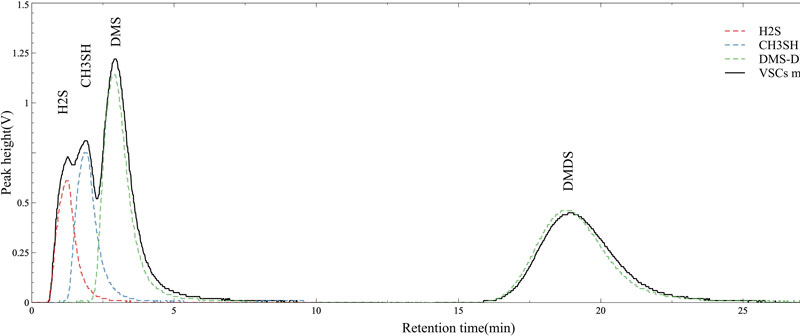
The chromatogram results of the GC/MOS system equipped with an MOS sensor as a detector were used to confirm that each VSC component could be separated at different RTs, and the reliability of repeated measurements was investigated by varying the concentration of the VSC standard gas. Fig. 4 shows the chromatograms obtained using H2S, CH3SH, DMS, and DMDS standard gases at a concentration of 1.0 ppm each. At a carrier gas flow rate of 6 mL/min and a column temperature of 30oC, we found that the VSC components were well separated and detected. The chromatogram results shown in Fig. 4 show that H2S, which has the lowest boiling point (bp) of -60oC among the standard gases, separated in the fastest time and was detected by the MOS gas sensor, followed by CH3SH (bp = 6oC), DMS (bp = 37oC), and DMDS (bp = 110oC). The separation characteristics were similar to the results of Yang et al. using GC/FPD [12].
Therefore, it was indirectly confirmed that the GC/MOS system using MOS gas sensors could be used for the quantitative and qualitative analyses of H2S, CH3SH, DMS, and DMDS, which are representative VSC components.
In addition, when the concentration of VSC standard gas was analyzed at 0.1, 0.5, 1.0, 5.0, and 10.0 ppm, H2S was separated at approximately 1.22 min, CH3SH at approximately 1.87 min, DMS at approximately 2.87 min, and DMDS at approximately 18.80 min, respectively. The RT results are summarized in Table 2. Based on the variation in RT with concentration and component in Table 2, the relative standard deviation (RSD) for each component is approximately 3%, which can be assessed as a relatively stable separation characteristic. In particular, the significant %RSD deviations of the components with shorter RTs appeared to be due to arithmetic limitations imposed by the relatively short RTs, which resulted in large deviations for small differences. Even when the concentration of VSC components increased, the detection times of each component, i.e., RTs of approximately 1.2 min, 1.8 min, 2.8 min, and 18.8 min, were clearly distinguishable. The separation characteristics of the GC/MOS system were found to be excellent. We have demonstrated the applicability of a new system that can distinguish gas components while utilizing a single MOS gas sensor as a detector.
3.2 Separation Characteristics as a Function of VSC Component Concentration
Fig. 5 illustrates the separation characteristics of the GC/MOS system as a function of the concentration of the individual VSC components, and Fig. 5 (a) shows the chromatograms obtained by sequentially increasing the concentration of the H2S standard gas to 0.1, 0.5, 1.0, 5.0, and 10.0 ppm. Similarly, Fig. 5 (b) shows the chromatogram results for the CH3SH standard gas, and Fig. 5 (c) shows the chromatogram results for the DMS and DMDS standard gases.
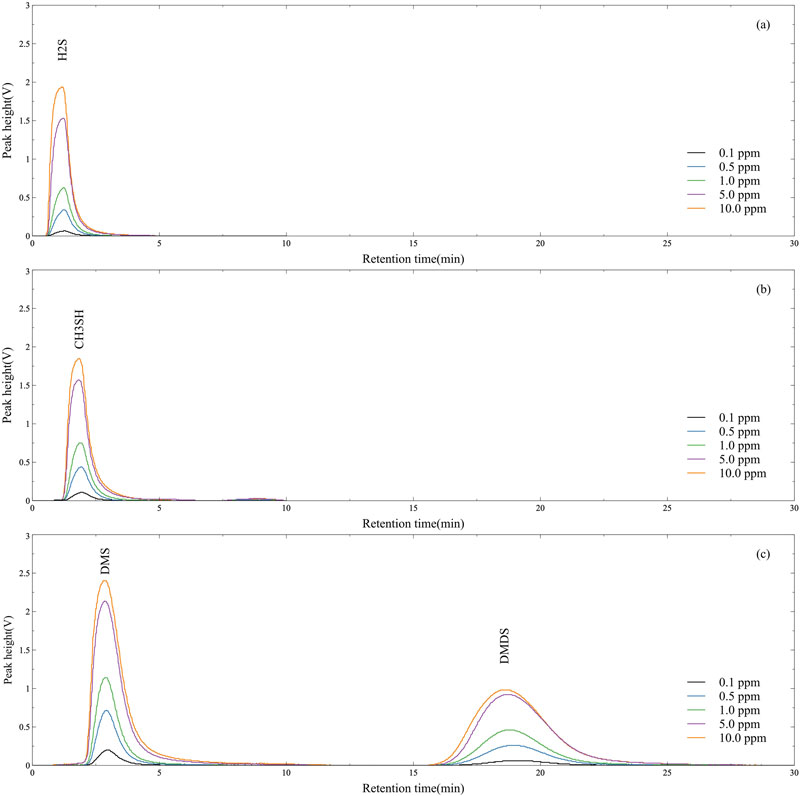
Chromatogram of the GC/MOS system with varying VSC concentrations. The (a), (b) and (c) are output peak value about H2S, CH3SH, DMS and DMDS standard gases, respectively.
The x-axis of the chromatogram represents the RT of the detected VSC components traveling through the column, and the y-axis represents the output (voltage) value of the MOS gas sensor. The maximum output value of the MOS gas sensor increased linearly with the concentration of H2S, and the maximum output value was detected at approximately 1.2 min. Moreover, as the concentration of CH3SH increased, the maximum output value of the MOS gas sensor increased proportionally, and the RT of the maximum output value was approximately 1.8 min.
Chromatograms obtained by analyzing a mixture of DMS and DMDS at 0.1, 0.5, 1.0, 5.0, and 10.0 ppm also show that the maximum output value of the MOS gas sensor increases with the concentration of the standard gas, with the maximum output value for DMS being approximately 2.8 min and for DMDS approximately 18.8 min, depending on the concentration of the standard gas. Consequently, the RT remained constant for each component.
Table 3 summarizes the results of five repetitions of the chromatogram analysis with different concentrations of H2S, CH3SH, DMS, and DMDS standard gases. The maximum output of the MOS gas sensor measured five times under the same concentration conditions varies by concentration, and the %RSD deviation of the components across the five concentration classes and five repetitions is 1.05 to 2.99 for H2S, 0.52 to 6.14 for CH3SH, 0.28 to 1.44 for DMS, and 1.15 to 5.28 for DMDS, respectively.
The GC/MOS system used in this study was configured to be automatically controlled by a PC operating program such that the diluted standard gas sample bag was automatically aspirated five times when connected to the sample inlet. It is believed that 4 mL of the standard gas sample was aspirated and left connected to the sample inlet without shaking the sample bag to mix for a 30 min analysis time. The repeatability of the GC/MOS system is expected to improve if more attention is paid to sample injection. As shown in Table 3, the maximum output of the MOS gas sensor increased proportionally with the concentration of the individual VSC components.
The correlations among these parameters are shown in Fig. 6. Fig. 6 (a) shows the concentration correlation of the H2S components separated using the GC/MOS system. The H2S concentration correlation formula for the maximum output value of the MOS sensor was calculated as y = 0.162 e2.23x, where x represents the maximum output value of the MOS gas sensor and y is the concentration of the VSC component, with a correlation coefficient (R2) of 0.991, showing a very high correlation. The correlation between the maximum output of the MOS gas sensor and the concentration of the CH3SH component in Fig. 6 (b) was y = 0.114 e2.66x, with a high correlation coefficient of 0.997. The correlation equation for DMS in Fig. 6 (c) is y = 0.091 e1.96x with a correlation coefficient of 0.994, and that for DMDS in Fig. 6 (d) is y = 0.104 e4.67x with a correlation coefficient of 0.998. Overall, the concentration correlation between the VSC component and the MOS sensor output of the GC/MOS system was excellent. Therefore, it is easy to calculate the maximum output value of the MOS sensor extracted from the results of the chromatograms analyzed using the GC/MOS system by converting the concentration of each VSC component.
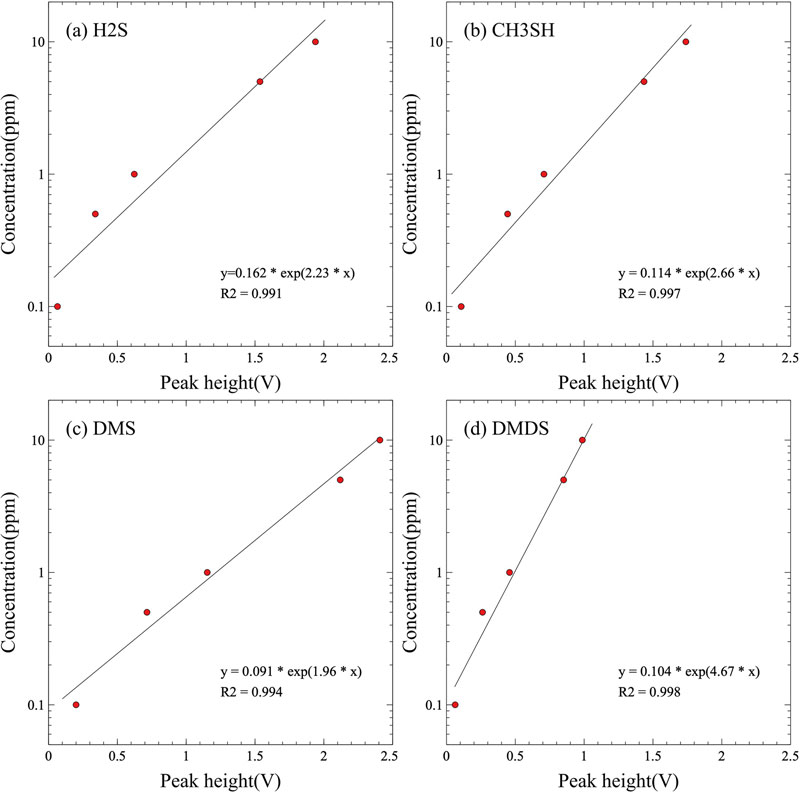
Correlation between the VSC concentration and the output value of the GC/MOS system (the peak height (V) is the output of the load resistor in the voltage divider circuit). The (a), (b), (c) and (d) are the concentration correlation curve about H2S, CH3SH, DMS and DMDS standard gases, respectively.
The chromatogram results of the VSC components analyzed using the above GC/MOS system revealed that individual VSC components with different RTs could be easily separated, and quantitative/qualitative analysis of each component was possible. In particular, the results are expected to be extended to the new concept of an electronic nose system that combines the advantages of MOS gas sensors that respond to different gas components using GC separation technology.
3.3 Separation Characteristics of VSCs for Mixed Components
The separation properties of the individual VSC components were analyzed in a mixture of components to investigate whether the GC/MOS system could effectively separate the VSC components, and the concentration of each component was quantified in a standard gas containing a mixture of four VSC components. The mixed standard gases were prepared by diluting the H2S, CH3SH, DMS, and DMDS components to a concentration of 1.0 ppm each in 3 L odorless bags in the same way as the diluted individual VSC components. The source components for all VSC standard gases were supplied by the manufacturer at approximately 100 ppm and diluted 100-fold based on the source component concentration to reach an arithmetic theoretical concentration of approximately 1.0 ppm.
Fig. 7 shows the chromatogram of a standard gas containing a mixture of H2S, CH3SH, DMS, and DMDS analyzed using the GC/MOS system, and the separation characteristics of the VSC components were compared by displaying the results in Fig. 4, which analyzed the individual components as a background (colored dotted lines). The results shown in the background are also from the same 1.0 ppm diluted standard gas; only the difference in maximum output for the H2S component can be seen, while the RTs of the other components are almost identical.
We also compared the arithmetic theoretical concentrations with the concentrations converted from the maximum output values for each VSC component of the chromatogram shown in Fig. 7. Table 4 summarizes the results of the chromatograms measured in triplicate with the mixed standard gas. The concentrations of each component were calculated by applying the maximum output value of each component to the concentration correlation equation in Fig. 6. The theoretical concentration of all VSC components is 1.0 ppm, and the concentrations of VSC components analyzed by the GC/MOS system were calculated in the range of 0.84 to 1.06 ppm. For H2S, the calculated concentrations were approximately 0.84 ppm, CH3SH was approximately 1.06 ppm, DMS was approximately 0.99 ppm, and DMDS was approximately 0.91 ppm. However, the deviation between the theoretical and actual analyzed concentrations of the H2S component was relatively large among the mixed standard gases. The maximum output value of the MOS gas sensor corresponding to 1.0 ppm in the H2S concentration correlation equation shown in Fig. 6 (a) is also far from the calculated value. Therefore, a concentration correlation equation that can reduce the error generated when the concentration correlation equation of the MOS gas sensor is applied to 1.0 ppm of H2S must be derived.
In addition, by comparing the separation characteristics of the gas components of the GC/MOS system as a detector and the GC/SAW system proposed by Yoo et al., it can be concluded that the GC/MOS system separates the peaks of the chromatogram more clearly. Although it is difficult to directly compare the separation characteristics of the results for acetone, ethanol, and methanol in Yoo et al.'s study, the selectivity of individual components separated by the column is determined to be low, and the GC/SAW system has a clear limitation as it cannot distinguish between ethanol and methanol when mixed [13].
Therefore, it was confirmed that the GC/MOS system was sufficiently capable of quantitatively/qualitatively analyzing VSC components, and it is expected that the separation characteristics of various odorous components other than VSC components can be studied to provide basic data for the application of a new electronic nose system.
3. CONCLUSIONS
A GC/MOS system utilizing the separation technology of GC columns and a MOS gas sensor as a detector was developed for the quantitative and qualitative analysis of VSC components. Specifically, the representative volatile sulfide compounds H2S, CH3SH, DMS, and DMDS were separated, and their individual concentrations were calculated, leading to the following conclusions:
- The GC/MOS system can separate H2S, CH3SH, DMS, and DMDS in VSCs. Each component that passed through the column was separated into clearly defined peaks with different RTs.
The repeatability of the RT, which is the peak separation characteristic of H2S, CH3SH, DMS, and DMDS analyzed by the GC/MOS system, showed relatively stable separation characteristics within approximately 3% RSD, although there were differences in some deviations by component. In addition, the repeatability of the peak output of the MOS gas sensor varied by component and concentration in the range of 1%–6% RSD.
- The maximum output of the MOS gas sensor increases proportionally with the concentration of the VSC components. The correlation coefficient (R2) is approximately 0.99 or higher. Furthermore, the concentrations of the individual components can be calculated from the maximum output of the MOS gas sensor.
A comparison of the separation characteristics of the chromatograms of individual VSC components with the chromatograms of mixed VSC components confirmed that each VSC component was separated at approximately the same RT, and the concentrations of individual components calculated from the maximum output values of the separated peaks were similar to the theoretical concentrations.
- By maximizing the advantages of MOS gas sensors and compensating for the selectivity problem, which has been recognized as a disadvantage, GC separation technology has enabled individual qualitative and quantitative analyses of VSC components. Future research on various gases is expected to lead to the development of new electronic nose systems.
REFERENCES
-
O. F. Hong, E. Kabir, J. Susaya, and K. H. Kim, “Characteristics of major offensive odorants emitted from urban stormwater catch basins”, Anal. Sci. Technol., Vol. 23, No. 4, pp. 347-356, 2010.
[https://doi.org/10.5806/AST.2010.23.4.347]

- J. H. Kim, B. R. Jung, and G. Ok, “Analysis of odorous sulfur compounds in air by GC/MS”, J. Environ. Anal. Health Toxicol., Vol. 9, No. 3, pp. 139-145, 2006.
- S. G. Seo, K. H. Jung, and J. M. Jeon, “Characteristics of sulfur compounds in Yeosu industrial complex”, J. Odor Indoor Environ., Vol. 2, No. 2, pp. 109-116, 2003.
- K. H. Kim, “The basic calibration properties of reduced sulfur compounds in air based on gas chromatographic approaches”, J. Odor Indoor Environ., Vol. 7, No. 3, pp. 170-178, 2008.
-
S. W. Han, C. S. Lee, H. S. Joo, K. C. Kim, S. Y. Kim, H. J. Ryu, J. M. Lee, H. S. Kim, and J. S. Han, “Performance assessment of H2S, NH3, and VOCs sensors for field application”, J. Odor Indoor Environ., Vol. 18, No. 3, pp. 261-271, 2019.
[https://doi.org/10.15250/joie.2019.18.3.261]

- J. W. Choi, M. K. Lee, C. W. Hong, J. H. Choi, M. K. Jang, K. B. W. R. Kim, G. E. Kim, G. R. Park, D. H. Ahn, and T. J. Nam, “Rapid freshness evaluation of mackerel scomber japoicus using sensor gas chromatography system”, Korean J. Fish. Aquatic. Sci., Vol. 50, No. 6, pp. 837-840, 2017.
-
M. Hanada, H. Koda, K. Onaga, K. Tanaka, T. Okabayashi, T. Itoh, and H. Miyazaki, “Portable oral malodor analyzer using highly sensitive In2O3 gas sensor combined with a simple gas chromatography system”, Anal. Chim. Acta, Vol. 475, No. 1-2, pp. 27-35, 2003.
[https://doi.org/10.1016/S0003-2670(02)01038-3]

-
H. S. Kim and S. J. Lee, “Determination of method detection limit for individual odorous substances using portable GC/MOS system”, J. Odor Indoor Environ., Vol. 22, No. 2, pp. 119-128, 2023.
[https://doi.org/10.15250/joie.2023.22.2.119]

-
H. S. Kim, Y. S. Park, and J. Y. Lee, “Quantitative and qualitative analysis method of odorous substances using a portable GC/MOS system”, J. Odor Indoor Environ., Vol. 18, No. 1, pp. 1-9, 2019.
[https://doi.org/10.15250/joie.2019.18.1.1]

-
S. Y. Oh, “Fast gas chromatography-surface acoustic waver sensor: An effective tool for discrimination and quality control of Lavandula species”, Sens. Actuators B Chem., Vol. 182, pp. 223-231, 2013.
[https://doi.org/10.1016/j.snb.2013.02.116]

-
E. J. Staples and S. Viswanathan, “Development of a novel odor measurement system using gas chromatography with surface acoustic wave sensor”, J. Air Waste Manag. Assoc., Vol. 58, No. 12, pp. 1522-1528, 2008.
[https://doi.org/10.3155/1047-3289.58.12.1522]

- S. B. Yang, M. S. Yu, and H. C. Hwang, “A study on the distribution of atmospheric concentrations of sulfur compounds by GC/FPD”, Anal. Sci. Adv., Vol. 16, No. 3, pp. 240-248, 2003.
-
B. K. Yoo, Y. W. Park, C. Y. Kang, S. J. Yoon, D. J. Choi, and J. S. Kim, “Surface acoustic wave gas sensors by assembling gas chromatography column”, J. Sens. Sci. Tech., Vol. 16, No. 1, pp. 39-43, 2007.
[https://doi.org/10.5369/JSST.2007.16.1.039]