Highly Selective and Sensitive Detection of Acetone by ZnWO4-WO3 Hetero-composite Spheres
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
ZnWO4-WO3 hetero-composite microspheres were prepared by ultrasonic spray pyrolysis of a solution containing Zn and W cations, followed by heat treatment at 600oC. The gas-sensing characteristics of 5 at% of Zn-added WO3 (5Zn-WO3; ZnWO4-WO3 hetero-composite) microspheres to 1 ppm acetone, ethanol, 20 ppm hydrogen (H2), 5 ppm carbon monoxide (CO), 25 ppb toluene, and 5 ppm ammonia (NH3) were measured at 325–400oC under 80% relative humidity (RH). The sensor using 5Zn-WO3 microspheres exhibited highly selective and sensitive gas-sensing properties to acetone at 375oC even under high humidity conditions. These superior gas-sensing properties were attributed to the increased resistance (electronic sensitization) through n-n heterojunction formation between WO3 and ZnWO4 phases and the acidic property of WO3, which exhibited a low gas response to interfering ethanol gas. The superior acetone gas-sensing characteristics of the 5Zn-WO3 sensor can be utilized in breath acetone analyzers for rapid, real-time ketogenic diet monitoring.
Keywords:
Gas sensor, Acetone, Hetero-composite, WO3, Breath analysis1. INTRODUCTION
Humans generally use carbohydrates as their primary energy source under normal conditions. However, when the carbohydrate intake is minimal, the body is forced to consume fat as an alternative energy source. When fat is catabolized during carbohydrate cut-off, acetone molecules are produced in the liver as a by-product of fatty acids. These diffuse into the blood and are finally released via human breath through gas exchange between the alveoli and capillaries [1-4]. Therefore, if breath acetone can be selectively and precisely detected through breath analysis, body fat loss can be effectively monitored. It is crucial for monitoring weight loss of obese patients.
Metal-oxide semiconductor (MOS) gas sensors have enabled the development of portable breath acetone analyzers owing to their superior miniaturization potential, simple gas-sensing mechanism, rapid response, and low cost. The gas-sensing characteristics of MOS can be tuned by modifying the material, such as tailoring the surface modification, doping, and nanostructure designs [5-7].
Different MOS gas sensors have been explored for the detection of acetone, including Si-WO3 nanoparticles [8], Rh-WO3 spheres [9], CeO2-In2O3 hollow spheres [10], and Tb-SnO2 yolk-shell spheres [11]. However, the development of acetone sensors is still in its infancy, and further research is required. Utilizing a heterocomposite as a sensing material is another strategy for designing highly selective and sensitive acetone gas sensors.
In this study, pure WO3 and 5at% Zn-added WO3 microspheres were prepared by ultrasonic spray pyrolysis with post-heat treatment. Their gas-sensing characteristics to 1 ppm acetone, ethanol, 20 ppm hydrogen (H2), 5 ppm carbon monoxide (CO), 25 ppb toluene, and 5 ppm ammonia (NH3) were evaluated at 325–400oC. The sensor using the Zn-added WO3 microspheres demonstrated high selectivity and sensitivity to acetone even under high humidity conditions (80% relative humidity [RH]), and its gas-sensing mechanism was discussed in relation to electronic sensitization by n-n heterojunction formation between WO3 and ZnWO4 and the acidic properties of WO3.
2. EXPERIMENTAL
2.1 Preparation of pure WO3 and 5Zn-WO3 microspheres
We prepared a 5at% Zn-added WO3 microsphere (5Zn-WO3) via ultrasonic spray pyrolysis, followed by heat treatment (Fig. 1). Next, 2.318 g of WO3 powders (WO3, powder, puriss., 99.9% Sigma-Aldrich Co., Ltd, USA), 0.110 g of Zn acetate dihydrate (Zn(CH3COO)2·2H2O, ACS reagent, ≥ 98%, Sigma-Aldrich Co., Ltd, USA), and 2.101 g of citric acid monohydrate (HOC(COOH)(CH2COOH)2·H2O, ACS reagent, ≥ 99%, Sigma-Aldrich Co., Ltd, USA) ([Zn]/[W] = 5at%) were dissolved in 200 mL of diluted ammonia solution (170 mL of D.I. water + 30 mL of 30% NH3 solution). The solutions were heated at 80oC with constant stirring until the solution became clear. Pure WO3 microspheres were prepared using the same procedure as Znadded WO3, but without adding Zn acetate dihydrate. The precursors of pure WO3 and Zn-added WO3 were annealed at 600oC for 2 h in a box furnace. For simplicity, 5at% Zn-added WO3 (ZnWO4-WO3 hetero-composite microspheres) is referred to as 5Zn-WO3. The morphology and phase of pure WO3 and 5Zn-WO3 microspheres were analyzed by field-emission scanning electron microscopy (FE-SEM; S-4300 Hitachi Co. Ltd., Japan) and X-ray diffraction (XRD; Rigaku Model/MAX-2500, Source: CuKα).
2.2 Measurement of gas-sensing characteristics
Pure WO3 and 5Zn-WO3 microspheres were mixed with terpinol base binders and screen printed on an alumina substrate with Au electrodes (size = 1.5 mm × 1.5 mm, thickness = 0.25 mm). The sensors were heat-treated at 400°C for 2 h in a box furnace to remove the organic compounds. Before gas sensor measurement, the sensors were annealed by a Ru-heater located in the backside of the substrate at 450oC for 3 h to increase the thermal stability of sensing materials. The sensing temperature (325–400oC) was controlled by adjusting the power of the Ruheater. The gas responses (S = RaRg-1 - 1: Ra: resistance in the air; Rg: resistance in gas) to 1 ppm acetone, ethanol, 20 ppm H2, 5 ppm CO, 25 ppb toluene, and 5 ppm NH3 were measured under a flow rate of 200 cm3 min−1 with RH 80%.
3. RESULTS AND DISCUSSION
3.1 SEM images
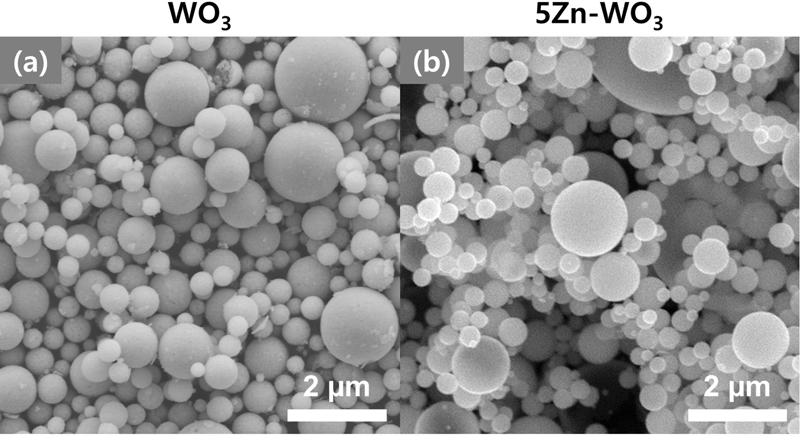
The morphologies of the pure and 5Zn-WO3 microspheres were observed using SEM (Fig. 2). Pure WO3 and 5Zn-WO3 microspheres exhibited similar size distributions and spherical morphologies with no distinct aggregation. In addition, the two specimens were composed of densely or thickly shelled spheres, which comprised nanosized primary particles.
3.2 Phase analysis
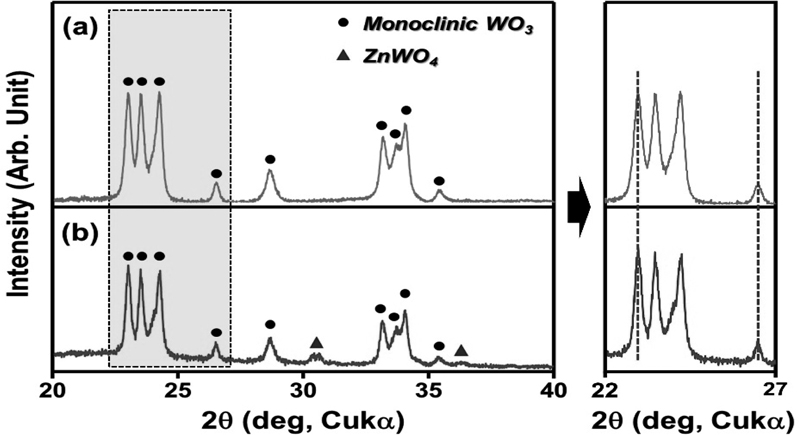
The phases of pure and 5Zn-WO3 microspheres were analyzed by X-ray diffraction (Fig. 3). Pure WO3 microspheres displayed only the γ-WO3 phase (JCPDS #43-1035), a stable phase under room and gas-sensing temperatures [12]. 5Zn-WO3 microspheres exhibited mixed phases of γ-WO3 and ZnWO4 phases. It can be attributed to sub-atomic scale uniform mixing of Zn and W cations via ultrasonic spray pyrolysis and relatively high annealing temperature (600oC) [13]. In addition, no significant shift in the WO3 peaks was observed, suggesting that almost all Zn cations were used for ZnWO4 formation without doping the WO3 lattice.
3.3 Gas-sensing characteristics and discussion
The gas-sensing characteristics of sensors using pure and 5Zn-WO3 microspheres to 1 ppm acetone, ethanol, 20 ppm H2, 5 ppm CO, 25 ppb toluene, and 5 ppm NH3 were measured at 325–400oC (Fig. 4). The sensing characteristics at < 325oC and > 400oC were not measured because the kinetics of gas sensing and recovery of < 325oC region were extremely slow, and the high temperature at > 400oC is unsuitable for long-term sensor operation and MEMS application. All sensors exhibited the typical characteristics of n-type metal oxide semiconductor gas sensors: a decrease in sensor resistance upon exposure to a reducing gas, which returned to its baseline upon exposure to air. Therefore, the gas response (S) was defined as RaRg-1 - 1 (Ra: resistance in the air, Rg: resistance in analyte gas).
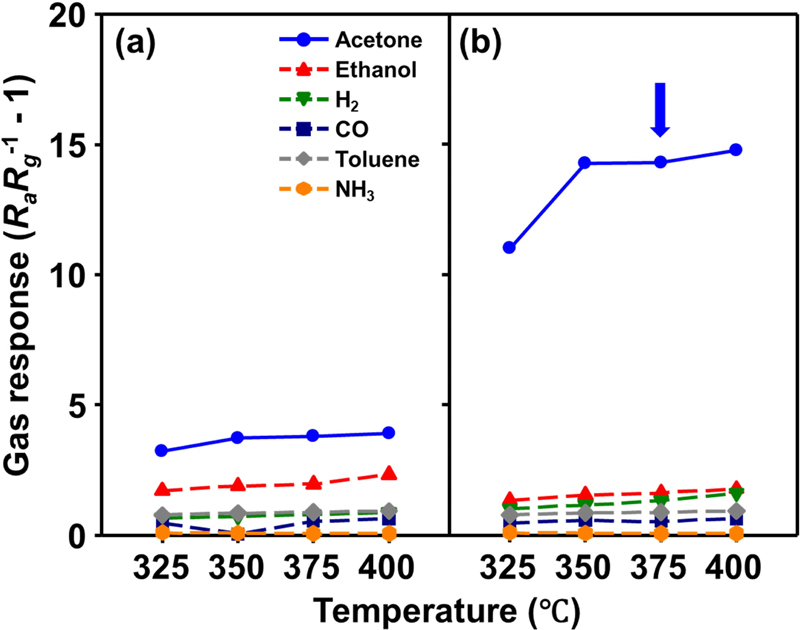
Gas-sensing characteristics of (a) pure and (b) 5Zn-WO3 microspheres to 1 ppm acetone, ethanol, 20 ppm H2, 5 ppm CO, 25 ppb toluene, and 5 ppm NH3 at 325–400oC and RH 80%
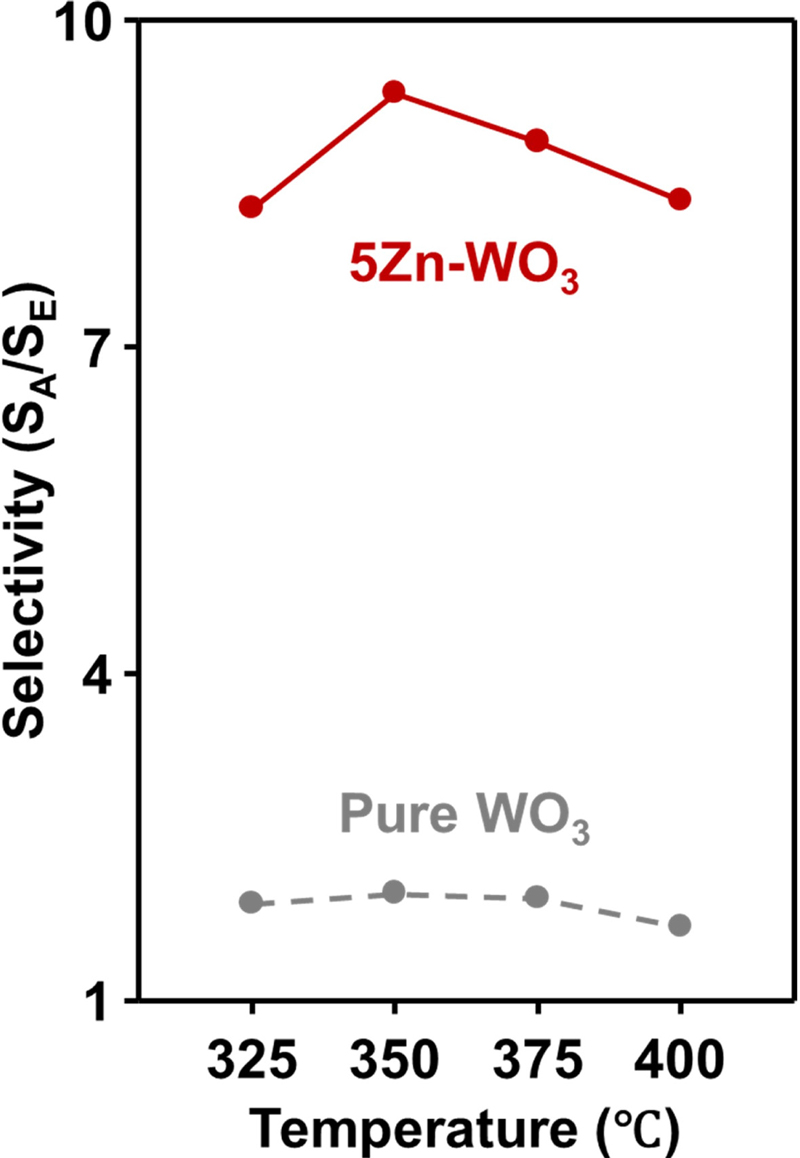
Sensors using pure WO3 microspheres exhibited elevated gas responses with increased temperature. In addition, pure WO3 microspheres exhibited the highest response to acetone gas, secondarily high gas responses to ethanol, and a relatively negligible gas response to other analyte gases. However, pure WO3 microspheres still exhibit relatively low selectivity to acetone (1.67–1.97), which is insufficient for breath acetone analyzer application. In contrast, 5Zn-WO3 microspheres exhibited a higher gas response to acetone than pure WO3 microspheres, even under high humidity conditions (80%). In addition, the selectivity values were greatly increased with Zn addition to WO3 (8.3–9.3) (Fig. 5). Considering gas response, selectivity, and responding speeds, the optimum gas-sensing temperature of 5Zn-WO3 was 375oC.
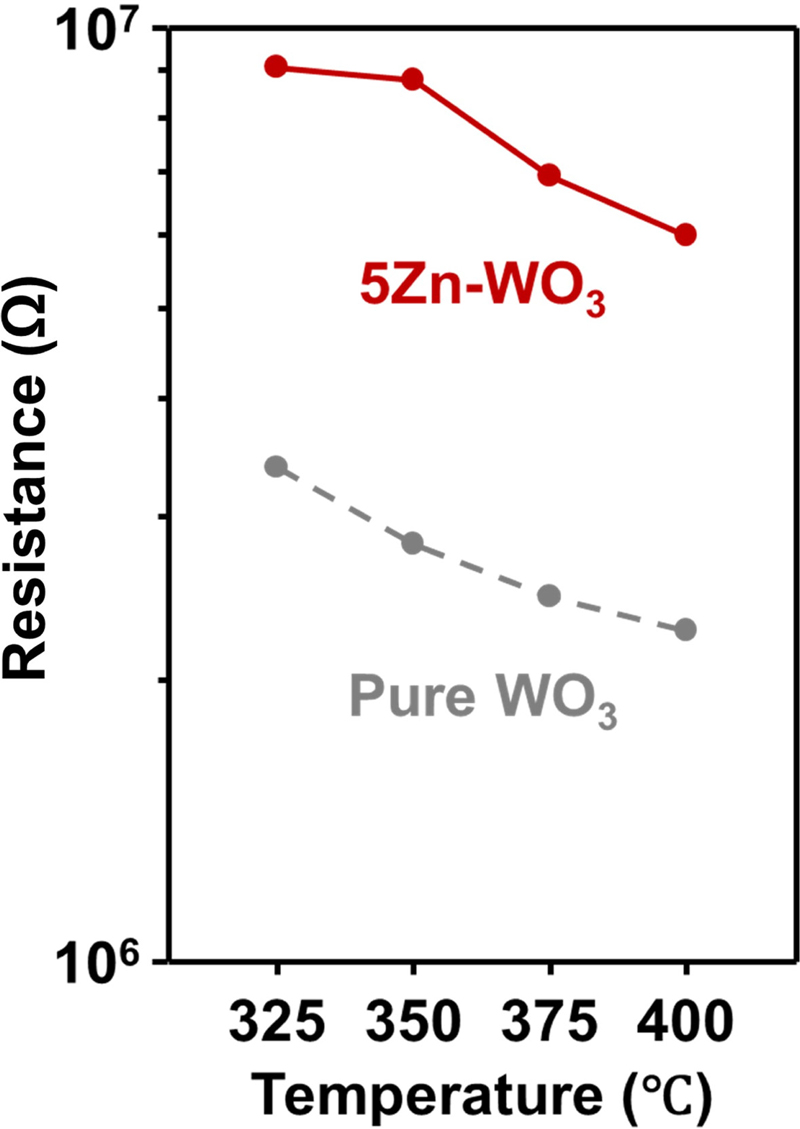
The increase in gas response and selectivity of 5Zn-WO3 can be understood in relation to the n-n heterojunction formation between ZnWO4 and WO3. The interface between ZnWO4 and WO3 extended the electron-depletion layer of WO3 by transferring electrons from WO3 to ZnWO4 and increasing the resistance of WO3 [14]. The 5Zn-WO3 microspheres exhibited a higher resistance than pure WO3 microspheres in all sensing temperature ranges (Fig. 6). This increase in resistance can be considered a major reason for the enhancement of the gas response because a sensor with a low background charge-carrier concentration exhibits a larger chemo-resistive variation if a fixed amount of charge carriers is injected into the gas sensor via a gas-sensing reaction (electronic sensitization) [15]. Several previous studies have reported that heterojunction formation significantly enhances gas responses. For example, NiWO4-NiO [16], NiMoO4-NiO [17], and Fe2O3-ZnFe2O4 [18] have demonstrated significant increases in response to analyte gases.
However, the resistance increase in WO3 can only explain the increase in the acetone response; high selectivity to acetone cannot be solely explained by the resistance increase. It is attributed to the acidic property of WO3 as well. WO3 has been reported to be an acidic metal oxide semiconductor that can convert reactive ethanol to less reactive ethylene via a dehydration reaction [19]. Because of the high stability of ethylene, it cannot undergo gas-sensing reactions with the sensing material surface, and gas responses to ethanol did not increase compared with those to acetone. Therefore, the increase in selectivity can be explained by the acidic properties of WO3.
Acetone is a key gas used to evaluate the effectiveness of body fat loss in obese patients. For instance, breath acetone concentrations increase to 2–5 ppm when the body fat decomposition rate reaches 2 g h−1. Considering the concentration of acetone released from human breath, the breath analyzer consisting of 5Zn-WO3 used in this study can be used for monitoring body fat loss.
4. CONCLUSIONS
A highly selective and sensitive gas sensor for detecting breath acetone was designed using 5Zn-WO3 microspheres. The pure WO3 sensor exhibited neither high gas response nor selectivity for acetone. In stark contrast, adding 5at% Zn ions to WO3 formed a ZnWO4 phase with a major WO3 conduction path. The heterojunction between WO3 and ZnWO4 in 5Zn-WO3 significantly enhanced the gas response and selectivity to sub-ppm level acetone, even under high-humidity conditions, through electronic sensitization. In addition, the acidic properties of WO3 are suggested to be the reason for its high selectivity for acetone.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT, nos. 2021M3H4A3A02086430 and RS-2024-00454367). This work was also supported by the Human Resource Development Program for Industrial Innovation (P0017012), funded by the Ministry of Trade, Industry, and Energy (MOTIE), Korea.
References
-
A. Paoli, A. Rubini, J. S. Volek, and K. A. Grimaldi, “Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets”, Eur. J. Clin. Nutr., Vol. 67, No. 8, pp. 789-796, 2013.
[https://doi.org/10.1038/ejcn.2013.116]

-
J. C. Anderson, “Measuring breath acetone for monitoring fat loss: review”, Obes., Vol. 23, No. 12, pp. 2327-2334, 2015.
[https://doi.org/10.1002/oby.21242]

-
A. T. Güntner, J. F. Kompalla, H. Landis, S. J. Theodore, B. Geidl, N. A. Sievi, M. Kohler, S. E. Pratsinis, and P. A. Gerber, “Guiding ketogenic diet with breath acetone sensors”, Sens., Vol. 18, No. 11, pp. 3655, 2018.
[https://doi.org/10.3390/s18113655]

-
K. Musa-Veloso, S. S. Likhodii, and S. C. Cunnane, “Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals”, Am. J. Clin. Nutr., Vol. 76, No. 1, pp. 65-70, 2002.
[https://doi.org/10.1093/ajcn/76.1.65]

-
N. Barsan, D. Koziej, and U. Weimar, “Metal oxide-based gas sensor research: how to?”, Sens. Actuator B Chem., Vol. 121, pp. 18-35, 2007.
[https://doi.org/10.1016/j.snb.2006.09.047]

-
D. R. Miller, S. A. Albar, and P. A. Morris, “Nanoscale metal oxide-based heterojunctions for gas sensing: a review”, Sens. Actuator B Chem., Vol. 204, pp. 250-272, 2014.
[https://doi.org/10.1016/j.snb.2014.07.074]

-
N. Yamazoe and K. Shimanoe, “New perspectives of gas sensor technology”, Sens. Actuator B Chem., Vol. 138, No. 1, pp. 100-107, 2009.
[https://doi.org/10.1016/j.snb.2009.01.023]

-
M. Righettoni, A. Tricoli, and S. E. Pratsinis, “Thermally Stable, Silica-Doped ε-WO3 for Sensing of Acetone in the Human Breath”, Chem. Mater., Vol. 22, No. 10, pp. 3152-3157, 2010.
[https://doi.org/10.1021/cm1001576]

-
K.-I. Choi, S.-J. Hwang, Z. Dai, Y. C. Kang, and J.-H. Lee, “Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics”, RSC Adv., Vol. 4, No. 95, pp. 53130-53136, 2014.
[https://doi.org/10.1039/C4RA06654E]

-
J.-W. Yoon, J.-S. Kim, T.-H. Kim, Y. J. Hong, Y. C. Kang, and J.-H. Lee, “A new strategy for humidity independent oxide chemiresistors: dynamic self?refreshing of In2O3 sensing surface assisted by layer?by?layer coated CeO2 nanoclusters”, Small, Vol. 12, No. 31, pp. 4229-4240, 2016.
[https://doi.org/10.1002/smll.201601507]

-
C.-H. Kwak, T.-H. Kim, S.-Y. Jeong, J.-W. Yoon, J.-S. Kim, and J.-H. Lee, “Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk–shell spheres for real-time breath analysis”, ACS Appl. Mater. Interfaces, Vol. 10, No. 22, pp. 18886-18894, 2018.
[https://doi.org/10.1021/acsami.8b04245]

-
S. Tanisaki, “On the phase transition of tungsten trioxide below room temperature, and configurational tuning of Au catalysts”, J. Phys. Soc. Jpn., Vol. 15, No. 4, pp. 566-573, 1960.
[https://doi.org/10.1143/JPSJ.15.566]

-
J.-S. Kim, K. B. Kim, H.-Y. Li, C. W. Na, K. Lim, Y. K. Moon, J. W. Yoon, and J.-H. Lee, “Pure and Pr-doped Ce4W9O33 with superior hydroxyl scavenging ability: humidity-independent oxide chemiresistors”, J. Mater. Chem. A, Vol. 9, No. 30, pp. 16359-16369, 2021.
[https://doi.org/10.1039/D1TA02618F]

-
P. Hu, J. Chen, Q. Ma, J. Yin, D. Zhou, C. Kou, J. Xu, and J. Xu, “One-step thermal compensation decomposition synthesis of ZnWO4/WO3 composite with synergy of multiple structural effects for efficient trace H2S detection”, Sens. Actuator B Chem., Vol. 381, p. 133388, 2023.
[https://doi.org/10.1016/j.snb.2023.133388]

- N. Yamazoe, G. Sakai, and K. Shimanoe, “Oxide semiconductor gas sensors”, Catal. Surv. Asia, Vol. 7, No. 1, pp. 63-75, 2003.
-
T.-H. Kim, C.-H. Kwak, and J.-H. Lee, “NiO/NiWO4 Composite Yolk–Shell Spheres with Nanoscale NiO Outer Layer for Ultrasensitive and Selective Detection of Sub ppm-level p-Xylene”, ACS Appl. Mater. Interfaces, Vol. 9, No. 37, pp. 32034-32043, 2017.
[https://doi.org/10.1021/acsami.7b10294]

-
B.-Y. Kim, J. H. A, J.-W. Yoon, C.-S. Lee, Y. C. Kang, F. Abdel-Hady, A. A. Wazzan, and J.-H. Lee, “Highly Selective Xylene Sensor Based on NiO/NiMoO4 Nanocomposite Hierarchical Spheres for Indoor Air Monitoring”, ACS Appl. Mater. Interfaces, Vol. 8, No. 50, pp. 34603-34611, 2016.
[https://doi.org/10.1021/acsami.6b13930]

-
J. F. Chan, J. K. Jeon, Y. K. Moon, and J.-H. Lee, “Highly sensitive xylene sensors using Fe2O3-ZnFe2O4 composite spheres”, J. Sens. Sci. Technol., Vol. 30, No. 4, pp. 191-195, 2021.
[https://doi.org/10.46670/JSST.2021.30.4.191]

-
T. Jinkawa, G. Sakai, J. Tamaki, N. Miura, and N. Yamazoe, “Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides”, J. Mol. Catal. A Chem., Vol. 155, No. 1-2, pp. 193-200, 2000.
[https://doi.org/10.1016/S1381-1169(99)00334-9]