Enhanced Acetone Detection in High-humidity Environments Using Cr-doped ε-WO3 Spheres
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Exhaled breath analysis has emerged as a non-invasive and cost-effective approach in medical diagnostics, particularly for detecting biomarkers such as acetone. This study presents the synthesis and characterization of Cr-doped ε-WO3 spheres designed to improve gas-sensing performance. These spheres were synthesized via ultrasonic spray pyrolysis, resulting in a material with a complex composition exhibiting high sensitivity and selectivity towards acetone over ethanol (response ratio = 13.2 at 325oC). The enhanced acetone sensitivity of the Cr-doped WO3 sensor is attributed to the strong interaction between the spontaneous electric dipole moment of ε-WO3 and the significant dipole moment of acetone. This sensor can detect exhaled acetone, facilitating effective monitoring of a ketogenic diet.
Keywords:
Gas sensors, Oxide semiconductors, ε-WO3, Acetone, Cr-doped WO31. INTRODUCTION
Detecting specific biomarker gases in exhaled breath has gained substantial importance in medical diagnostics due to its cost-effectiveness and non-invasive approach [1-5]. Exhaled breath comprises a complex mixture of gaseous, including volatile organic compounds (VOCs), present in concentrations ranging from parts per billion (ppb) to parts per million (ppm), often under humid conditions. Precisely detecting specific VOCs in exhaled breath can provide critical insights into various medical conditions [6]. Particularly, acetone (CH3COCH3) serves as a critical indicator for ketogenic diets, which are scientifically recognized for their effectiveness in weight management. Acetone concentrations in exhaled breath range from 2 to 5 ppm when the body fat decomposition rate reaches 2 g/h [7,8]. Therefore, developing compact breath acetone sensors is crucial for enabling routine monitoring, significantly reducing healthcare costs associated with diet management.
This study reports on a novel sensing material designed for portable breath acetone sensors based on Cr-doped ε-WO3 spheres. The material was synthesized using ultrasonic spray pyrolysis, which converts precursor solution droplets into oxide spheres with complex compositions [9,10]. Specifically, Cr-doped WO3 containing 5 at% Cr was synthesized from a solution of chromium nitrate nonahydrate and ammonium metatungstate hydrate, and its gas-sensing properties were systematically evaluated. The Cr-doped ε-WO3 spheres exhibited superior gas response and selectivity to acetone. This study primarily focused on identifying the critical parameters influencing gas-sensing performance and elucidating the underlying mechanisms responsible for the observed sensing behavior.
2. EXPERIMENTAL
2.1 Synthesis of Gas Sensing Materials
Cr-doped WO3 spheres were synthesized using ultrasonic spray pyrolysis. A spray solution was prepared by dissolving ammonium metatungstate hydrate ((NH4)6H2W12O4o·xH2O, 99.99%, Sigma-Aldrich, USA) and chromium nitrate (Cr(NO3)3·9H2O, 99.99%, Sigma-Aldrich, USA) in 100 mL of distilled water. The concentration of the cations, [W] + [Cr], was maintained at 0.1 M, with the molar ratio of Cr to W ions ([Cr]/([W] + [Cr])) set at 0.05. The spray solution was atomized using five ultrasonic transducers operating at a frequency of 1.7 MHz and directed into a high-temperature quartz tube (900oC; length: 1200 mm; diameter: 50 mm) with the assistance of a carrier gas (air, 10 L min-¹). The resulting precursor powders were captured in a Teflon bag filter and converted into pure WO3 and Cr-doped WO3 spheres by annealing at 600oC for 2 h in air. The Cr-doped WO3 spheres, containing 5 at% chromium, are referred to as 5Cr-WO3.
2.2 Fabrication of Sensing Film
The 5Cr-doped WO3 spheres were mixed with an organic binder (FCM, a terpineol-based ink vehicle, USA) in a weight ratio of 30:70 to produce a slurry. Subsequently, the slurry was screen-printed onto an alumina substrate (area: 1.5 × 1.5 mm²; thickness: 0.25 mm), which was pre-coated with two Au electrodes on the top surface and a micro-heater on the bottom. After screen printing, the sensors were subjected to heat treatment in air at 450oC for 2 h to remove the organic binder and stabilize the sensing film.
2.3 Characterization of Gas Sensing Properties
The micro-heater on the alumina substrate controlled the sensing temperature, with measurements taken between 300 and 400oC. The relative humidity (RH) of the gas was maintained at 80%. The gas response was evaluated using the formula S = (Ra/Rg) – 1, where Ra represents air resistance and Rg denotes the resistance in the target gas. Performance was assessed based on these gas response values, with two-probe DC resistance measurements conducted using an electrometer connected to a computer.
3. RESULTS AND DISCUSSIONS
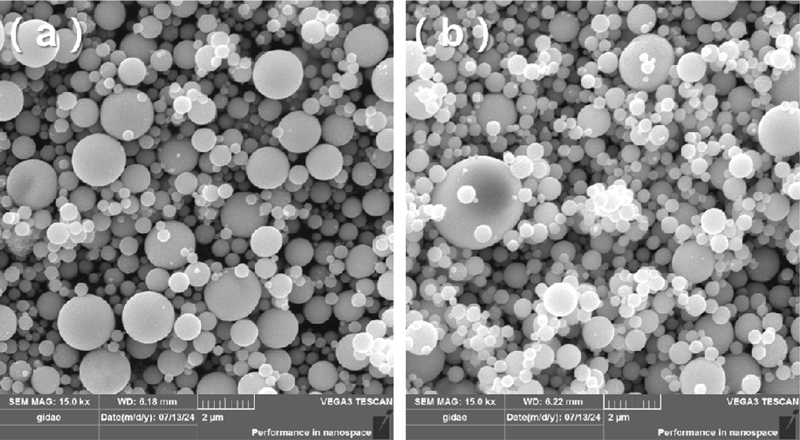
Pure WO3 and 5Cr-WO3 spheres were synthesized using ultrasonic spray pyrolysis, followed by heat treatment at 600oC for 2 h. The spheres exhibited a spherical morphology (Fig. 1) with similar size distributions. Particle size distributions were assessed by measuring the diameter of 100 spheres per sample. The average diameters of pure WO3 and 5Cr-WO3 spheres were 0.66 ± 0.30 μm and 0.73 ± 0.38 μm, respectively.
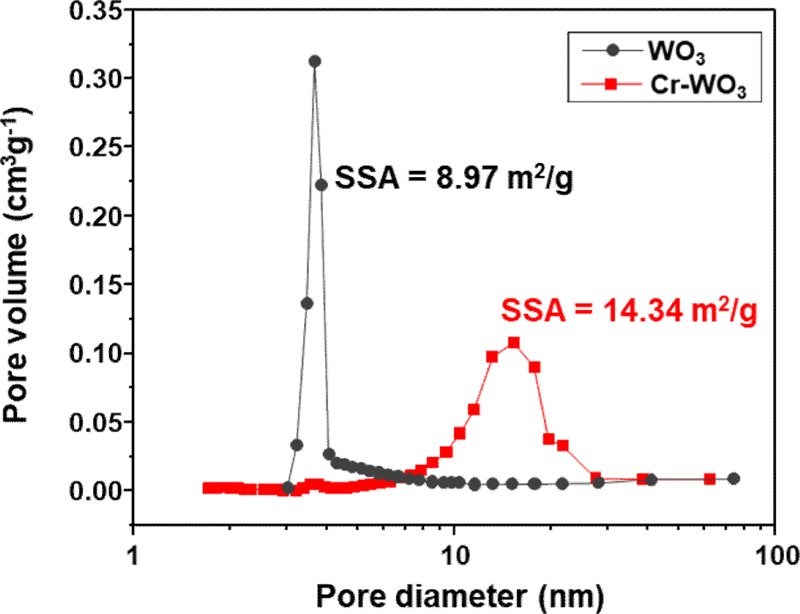
The pore size distributions and specific surface areas of pure and 5Cr-WO3 spheres were analyzed using Brunauer-Emmett-Teller (BET) analysis (Fig. 2). The specific surface areas for pure WO3 and 5Cr-WO3 were 8.97 m²/g and 14.34 m²/g, respectively. The crystallite sizes of pure WO3 and 5Cr-WO3spheres were calculated as 42.0 and 21.5 nm, respectively, using Scherrer’s equation. The increase in specific surface area observed with Cr doping is attributed to the reduction in the crystallite size, as confirmed by X-ray diffraction (XRD) analysis. Additionally, the number of mesopores (2–50 nm), which enhances gas accessibility, increased with Cr doping. These findings indicate that the structural modifications resulting from Cr doping are beneficial for improving gas accessibility in the sensing material
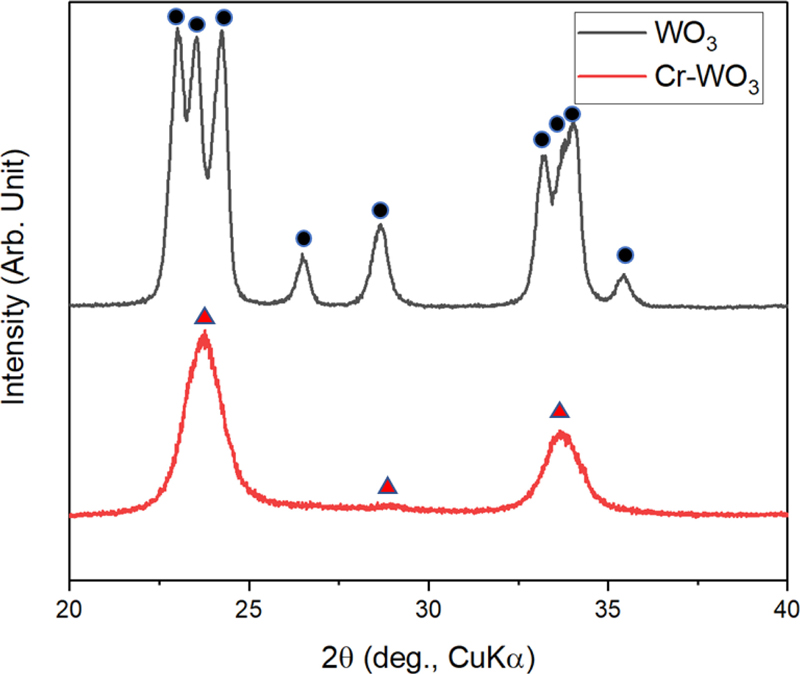
XRD analysis revealed the phases present in the samples (Fig. 3). The WO3 spheres were identified as γ-WO3 (JCPDS# 83-0950). The 5Cr-WO3 sphere displayed broad XRD peaks deconvoluted into the ε-WO3 phase (JCPDS# 87-2402). No secondary Cr-related phase was detected in the 5Cr-WO3 samples (Fig. 3), indicating that Cr ions were incorporated into the W sites. Given that ε-WO3 is stable only below –40oC and maintaining this phase at higher temperatures (e.g., sensor operating temperatures) is challenging, Cr doping is an effective strategy for stabilizing ε-WO3. Wang et al. demonstrated that ferroelectric ε-WO3 nanoparticles exhibited high selectivity and sensitivity towards acetone. This enhancement was achieved by introducing Cr into the non-acentric γ-WO3 structure, resulting in the acentric ε-WO3 phase. The improved sensing performance for acetone is attributed to the preferential adsorption of acetone molecules onto the polarized ferroelectric domains on the surface [11]. The high polarity of Acetone, with a dipole moment of 2.88 D, significantly exceeded that of major interfering breath gases such as ethanol (1.70 D) [12]. However, the agglomerated nanoparticle form of Cr-doped ε-WO3 showed inadequate gas response and selectivity toward acetone detection due to its limited specific surface area and active sites. Therefore, the formation of nanostructured Cr-doped ε-WO3 is anticipated to enhance the gas-sensing properties of 5Cr-WO3 material. Ultrasonic spray pyrolysis, acting as a reaction container for droplets, facilitates the production of well-defined 5Cr-WO3 spheres with consistent spherical morphology, offering a promising approach for designing high-performance gas sensors.
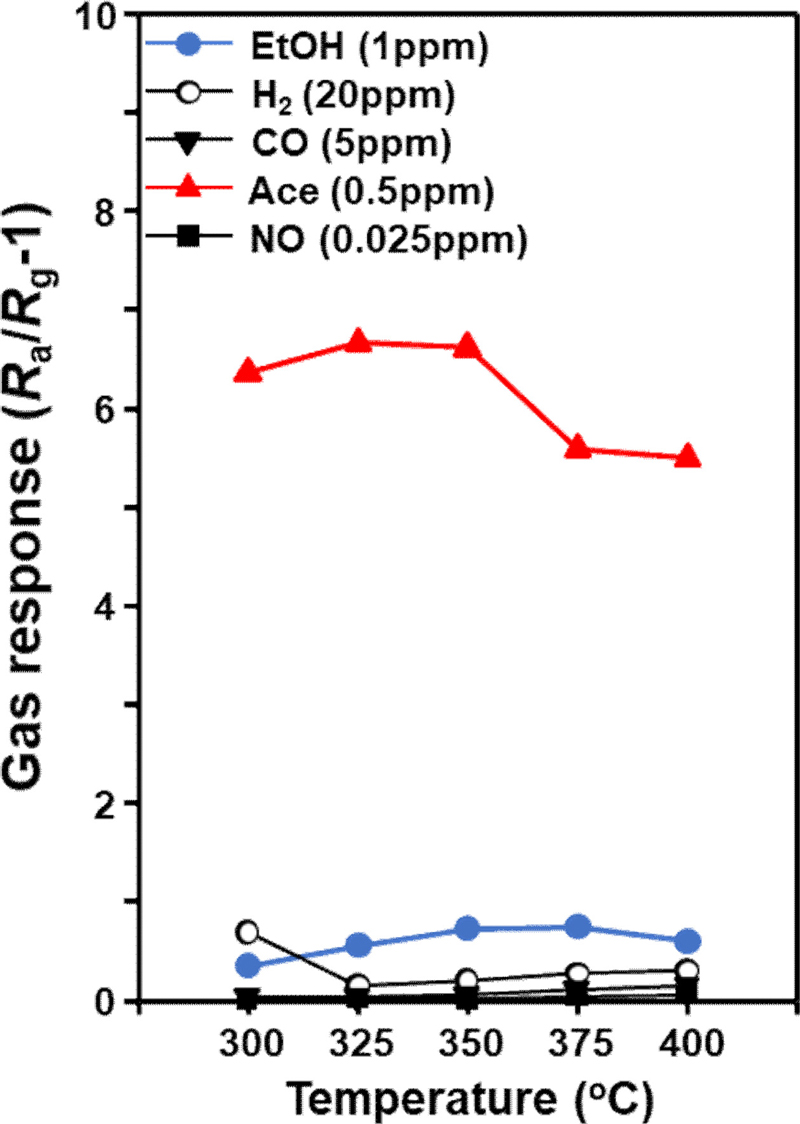
The gas sensing characteristics of 5Cr-WO3 spheres were evaluated in the presence of ethanol (EtOH), hydrogen (H2), carbon monoxide (CO), and nitric oxide (NO), all of which can be exhaled and potentially interfere with acetone (Ace) detection. Concentrations of these gases were chosen based on diagnostic levels reported in the literature: EtOH: 1 ppm, H2: 20 ppm, CO: 5 ppm, NO: 0.025 ppm, and Ace: 0.5 ppm. [13]. Measurements were conducted across a temperature range of 300oC to 400oC. All sensors exhibited n-type gas sensing behavior, characterized by a decrease in resistance upon exposure to reducing gases and a return to baseline resistance in the air.
The gas sensing performance of the pure WO3 sphere sensor, with a γ-WO3 phase, has shown relatively low responses to various analyte gases within the temperature range of 300oC to 400oC [12]. However, the 5Cr-WO3 sensor, composed of the ε-WO3 phase, demonstrated markedly enhanced gas sensing characteristics. Notably, the response to acetone at 325oC (S = 6.6) was significantly higher than ethanol (S = 0.5). The doping of Cr in WO3 led to a marked increase in acetone response, resulting in high selectivity and sensitivity (SA/SE = 13.2). The superior acetone sensing performance is attributed to the strong interaction between the electric dipole moment of ε-WO3 and the high dipole moment of acetone, coupled with the extensive reaction sites provided by the high surface area of 5Cr-WO3 n. This indicates that the nanostructured ε-WO3 materials are highly effective for acetone detection.
Acetone concentration in human breath correlates closely with fat metabolism, making acetone sensors instrumental in monitoring the efficacy of ketogenic diets (KD) by providing real-time feedback on fat burning. Given the global prevalence of obesity, with one-third of adults affected, acetone sensors have significant market potential. For individuals on a ketogenic diet, breath acetone levels range from 1.7 to 8.0 ppm [14]. Therefore, the sensor developed in this study, capable of detecting sub-ppm levels of acetone with high sensitivity and selectivity, is well-suited for monitoring and managing adherence to the ketogenic diet.
4. CONCLUSIONS
This study successfully developed Cr-doped ε-WO3 spheres as an effective sensing material for acetone detection in exhaled breath. The material, synthesized via ultrasonic spray pyrolysis, exhibited superior sensitivity and selectivity toward acetone, with a response ratio of 13.2 at 325oC. The enhanced performance of the 5Cr-WO3 sensor is attributed to the strong interaction between the spontaneous electric dipole moment of ε-WO3 and the high dipole moment of acetone. These results demonstrate that the 5Cr-WO3 sensor is a promising tool for non-invasive monitoring of acetone levels, especially for managing ketogenic diets.
Acknowledgments
This work was supported by the Korea Institute of Material Science (KIMS) internal R&D program (No. PNK9640).
References
-
K. B. Kim, M. S. Sohn, S. Min, J.-W. Yoon, J.-S. Park, J. Li, Y. K. Moon, and Y. C. Kang, “Highly Selective and Reversible Detection of Simulated Breath Hydrogen Sulfide Using Fe?Doped CuO Hollow Spheres: Enhanced Surface Redox Reaction by Multi?Valent Catalysts”, Small, Vol. 20, p. 2308963, 2024.
[https://doi.org/10.1002/smll.202308963]

-
J.-W. Yoon and J.-H. Lee, “Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: recent progress and future perspectives”, Lab. Chip, Vol. 17, No. 21, pp. 3537-3557, 2017.
[https://doi.org/10.1039/C7LC00810D]

-
H. G. Moon, Y. R. Choi, Y.-S. Shim, K.-I. Choi, J.-H. Lee, J.-S. Kim, S.-J. Yoon, H.-H. Park, C.-Y. Kang, and H. W. Jang, “Extremely Sensitive and Selective NO Probe Based on Villi-like WO3 Nanostructures for Application to Exhaled Breath Analyzers”, ACS Appl. Mater. Interfaces, Vol. 5, No. 21, pp. 10591-10596, 2013.
[https://doi.org/10.1021/am402456s]

-
A. T. Güntner, S. Abegg, K. Königstein, P. A. Gerber, A. Schmidt-Trucksäss, and S. E. Pratsinis, “Breath Sensors for Health Monitoring”, ACS Sens., Vol. 4, No. 2, pp. 268-280, 2019.
[https://doi.org/10.1021/acssensors.8b00937]

-
J. J. Park, J. Lee, G. H. Kim, J.-H. Kim, H.-S. Lee, and W. Lee, “Breath visualization: Colorimetric detection of acetone gas using ion-pairing dyes based on hollow silica particles”, Sens. Actuators B Chem., Vol. 406, p. 135373, 2024.
[https://doi.org/10.1016/j.snb.2024.135373]

-
S.-J. Kim, S.-J. Choi, J.-S. Jang, H.-J. Cho, and I.-D. Kim, “Innovative Nanosensor for Disease Diagnosis”, Acc. Chem. Res., Vol. 7, No. 7, pp. 1587-1596, 2017.
[https://doi.org/10.1021/acs.accounts.7b00047]

-
J. C. Anderson, “Measuring breath acetone for monitoring fat loss: review”, Obesity, Vol. 23, No. 12, pp. 2327-2334, 2015.
[https://doi.org/10.1002/oby.21242]

-
K. Musa-Veloso, S. S. Likhodii, and S. C. Cunnane, “Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals”, Am. J. Clin. Nutr., Vol. 76, No. 1, pp. 65-70, 2002.
[https://doi.org/10.1093/ajcn/76.1.65]

-
G. L. Messing, S.-C. Zhang, and G. V. Jayanthi, “Ceramic Powder Synthesis by Spray Pyrolysis”, J. Am. Ceram. Soc., Vol. 76, No. 11, pp. 2707-2726, 1993.
[https://doi.org/10.1111/j.1151-2916.1993.tb04007.x]

-
J.-S. Park, J. K. Kim, J. H. Hong, J. S. Cho, S.-K. Park, and Y. C. Kang, “Advances in the synthesis and design of nanostructured materials by aerosol spray processes for efficient energy storage”, Nanoscale, Vol. 11, No. 41, pp. 19012-19057, 2019.
[https://doi.org/10.1039/C9NR05575D]

-
L. Wang, A. Teleki, S.E. Pratsinis, and P.I. Gouma, “Ferroelectric WO3 nanoparticles for acetone selective detection”, Chem. Mater., Vol. 20, No. 15, pp. 4794-4796, 2008.
[https://doi.org/10.1021/cm800761e]

-
H. J. Choi, J.-H. Chung, J.-W. Yoon, and J.-H. Lee, “Highly selective, sensitive, and rapidly responding acetone sensor using ferroelectric ε-WO3 spheres doped with Nb for monitoring ketogenic diet efficiency”, Sens. Actuators B Chem., Vol. 338, p. 129823, 2021.
[https://doi.org/10.1016/j.snb.2021.129823]

-
S.-W. Park, S.-Y. Jeong, Y. K. Moon, K. B. Kim, J.-W. Yoon, and J.-H. Lee, “Highly Selective and Sensitive Detection of Breath Isoprene by Tailored Gas Reforming: A Synergistic Combination of Macroporous WO3 Spheres and Au Catalysts”, ACS Appl. Mater. Interfaces, Vol. 14, No. 9, pp. 11587-11596, 2022.
[https://doi.org/10.1021/acsami.1c19766]

-
A. T. Güntner, J. F. Kompalla, H. Landis, S. J. Theodore, B. Geidl, N. A. Sievi, M. Kohler, S. E. Pratsinis, and P. A. Gerber, “Guiding ketogenic diet with breath acetone sensors”, Sens., Vol. 18, No. 11, p. 3655, 2018.
[https://doi.org/10.3390/s18113655]