Colorimetric Sensor Based on Pd–MoO3 Nanowires for Hydrogen Gas Leak Detection
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The early detection of hydrogen gas leaks is crucial because of their high explosion risk. Current oxide–semiconductor-based hydrogen sensors are reliant on electrical circuits that may fail during accidents and require high temperatures, thereby raising safety concerns. Thus, there is an urgent need for the development of simpler and more intuitive sensors that can operate at room temperature. This study proposed a hydrogen sensor based on Pd–MoO3 nanowires. The sensor exhibited a visible color change upon exposure to hydrogen at room temperature. The Pd–MoO3 nanowires were synthesized by decorating the surface of hydrothermally produced MoO3 nanowires with 1–5 wt.% Pd. Upon exposure to 5% hydrogen gas at room temperature, all Pd–MoO3 nanowires exhibited distinct color changes (ΔE). In particular, the MoO3 nanowires with 3 wt.% Pd (3Pd–MoO3) yielded an exceptionally high ΔE value of over 15 within 10 min. Further, the 3Pd–MoO3 nanowires exhibited a noticeable color change (ΔE > 1.6) within 2 min, demonstrating their potential for highly sensitive and rapid hydrogen detection. The outstanding color change of the 3Pd–MoO3 nanowires was attributed to valence changes in both Mo (Mo6+ and Mo5+) and Pd (Pd2+ and Pd0) upon exposure to hydrogen.
Keywords:
Colorimetric sensor, Hydrogen detection, Pd–MoO3, Nanowires, Hydrothermal synthesis1. INTRODUCTION
Hydrogen energy is increasingly being recognized as a viable alternative to fossil fuels, with widespread applications in industries such as petrochemicals, semiconductors, refining, and fuel cells [1]. Recently, they have gained attention as a power source for mobility and power generation because of their high energy density (120 MJ/kg) [2]. However, hydrogen poses a significant explosion risk when its concentration in air exceeds 4% [3]. Moreover, its flames are extremely difficult to extinguish once ignited [4]. Therefore, careful monitoring of hydrogen gas leakage is crucial. Because hydrogen is a colorless and odorless gas that cannot be detected by humans, a detector is essential for identifying leaks.
Current hydrogen detectors such as gas chromatography-flame ionization detectors offer precise detection at low concentrations [5]. However, these detectors are bulky, expensive, and time consuming, thereby rendering them unsuitable for real-time monitoring. Oxide semiconductor gas sensors such as those made using SnO2, In2O3, and WO3 provide a compact and cost-effective alternative for real-time detection [6,7]. Nevertheless, their reliance on electric circuits and high operating temperatures (e.g., 200–400oC) serve as bottlenecks. Thus, there is need to develop new sensors capable of rapid and intuitive detection of hydrogen at room temperature.
Colorimetric gas sensors, which change color upon the interaction of their sensing materials with specific gases, can detect hydrogen without requiring complex electrical devices [8]. In addition, these sensors are safe because their room-temperature operability reduces the ignition risk. These advantages render colorimetric gas sensors viable candidates for early hydrogen leak detection.
Among the various colorimetric materials, MoO3 is particularly promising for hydrogen detection because of its ability to change color upon exposure to hydrogen [9]. For instance, Sun et al. [10] reported that MoO3 nanobelts changed color from light gray to dark blue in the presence of hydrogen. Pd is another favorable material for colorimetric hydrogen sensing because of its ability to change color in the presence of hydrogen [11]. The excellent colorimetric properties of MoO3 and Pd suggest that their combination can facilitate the development of highly effective colorimetric hydrogen sensors.
This study investigated the colorimetric hydrogen-sensing properties of Pd–MoO3 nanowires. Pd–MoO3 nanowires were prepared by decorating the surface of hydrothermally synthesized MoO3 nanowires with 1–5 wt.% Pd. Subsequently, a hydrogen sensor was fabricated by screen printing the nanowires onto a glass slide. All Pd–MoO3 sensors demonstrated colorimetric hydrogen-sensing properties at room temperature. Among the sensors, those containing 3 wt.% Pd exhibited a drastic color change from light brown to dark gray, which is not typical of MoO3 or Pd alone under hydrogen exposure. Therefore, the colorchange mechanism is discussed. This study aimed to demonstrate the potential of Pd–MoO3 for visible hydrogen detection and elucidate its colorimetric hydrogen-sensing mechanism.
2. EXPERIMENTAL
2.1 Synthesis of Pd–MoO3 nanowires
MoO3 nanowires were synthesized by dissolving 0.01 mol of Na2MoO4·2H2O (≥99.5%, Sigma-Aldrich, USA) and 7.25 ml of HNO3 (ACS reagent 70%, Sigma-Aldrich, USA) in 32.75 ml of distilled water. Subsequently, this solution was in an autoclave and subjected to hydrothermal treatment at 200oC for 12 h. To synthesize Pd–MoO3 nanowires, 0.2 g of MoO3 nanowires were dispersed in H2PdCl4 solutions of varying concentrations. A H2PdCl4 solution was prepared by dissolving PdCl2 (99%, Sigma-Aldrich, USA) in HCl (ACS reagent 35%, Sigma-Aldrich, USA) in a 1: 2 ratio. In particular, 0.2, 0.6, and 1.0 mg of PdCl2 were used at HCl concentrations of 0.2, 0.7, and 1.2 μL to achieve different Pd concentrations. The mixture was stirred for 30 min to ensure uniform Pd distribution on the MoO3 nanowire surface. Thereafter, the mixture was centrifuged five times with ethanol and distilled water, followed by drying at 40oC for 6 h to obtain Pd–MoO3 nanowires. For simplicity, the MoO3 nanowires decorated with 1, 3, and 5 wt.% Pd are referred to as 1Pd–MoO3, 3Pd–MoO3, and 5Pd–MoO3, respectively.
2.2 Fabrication of Pd–MoO3 colorimetric sensor
The colorimetric sensor was fabricated by mixing 0.03 g of Pd–MoO3 nanowires with 50 μL of a binder (α-terpineol, 90% technical grade, Sigma-Aldrich, USA) to form a slurry. This slurry was then screen-printed onto a glass slide (area = 10 mm × 30 mm) and then dried in an oven at 70oC for 2 h.
2.3 Characterization
The crystal structures of the Pd–MoO3 nanowires were analyzed by X-ray diffraction (XRD, SmartLab, Rigaku, Japan). The compositions and chemical states of the nanowires were confirmed via X-ray photoelectron spectroscopy (XPS, Sigma Probe, Thermo VG Scientific). The morphology and microstructure of the nanowires were observed through field-emission scanning electron microscopy (FE-SEM; S-4700, Hitachi, Japan) and spherical aberration-corrected transmission electron microscopy (TEM; JEM-ARM200F, JEOL, Japan).
2.4 Hydrogen sensing test
The colorimetric properties of the sensors in response to hydrogen were evaluated in a quartz chamber connected to a gas-supply system. The chamber atmosphere was controlled by flowing dry air (99.999%, Hankook Special Gases) and hydrogen gas (5%, Hankook Special Gases) using mass flow controllers (MFC) in the gas supply system. The flow rate was fixed at 500 cm3·min-1, and all measurements were conducted at room temperature (17±3oC). The color change of the sensor was monitored in real time using a smartphone camera. The intensity of the color change was quantified based on calculations of the color difference (ΔE) between two colors within the International Commission on Illumination (CIE) L*a*b* color space [12], using the following equation:
| (1) |
where L, a, and b are the lightness, red-green position, and yellow-blue position of the sample, respectively.
3. RESULTS AND DISCUSSIONS
3.1 Characterization of Pd–MoO3 nanowires
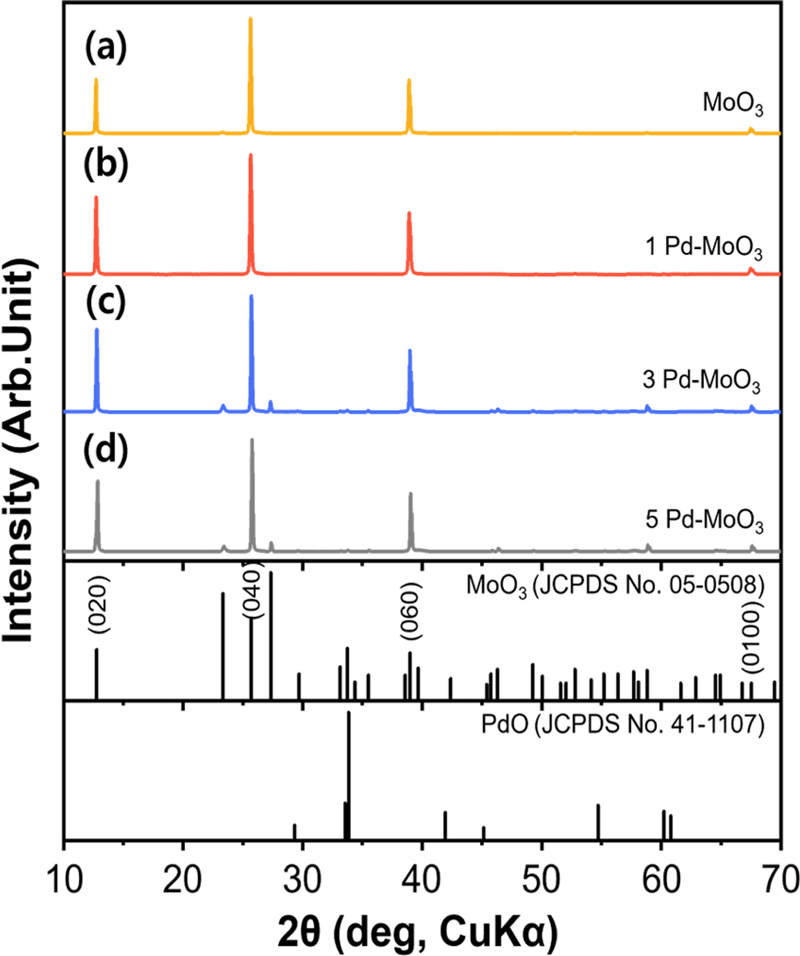
XRD analysis was performed to confirm the phases of the MoO3 and Pd–MoO3 nanowires (Fig. 1). All samples exhibited the orthorhombic phase of MoO3 (α-MoO3) (JCPDS #05-0508). The diffraction peaks were predominantly oriented along the (020), (040), and (060) directions, suggesting that the MoO3 crystals grew mainly along the b-axis during the hydrothermal process. Further, no Pd–related peaks were observed in any of the Pd–MoO3 samples, which may be because of the low concentration of Pd.
Fig. 2 (a–d) show SEM images of the MoO3 and Pd–MoO3 nanowires. The MoO3 nanowires had a diameter of approximately 0.5 μm and lengths ranging as 10–20 μm (Fig. 2 (a)). The Pd decoration did not significantly alter the overall wire-like morphology, although it might have slightly affected the surface texture and uniformity of the wires (Fig. 2 (b–d)). Both the SEM and TEM images confirmed the anisotropic growth of the nanowires, which was consistent with the XRD results.
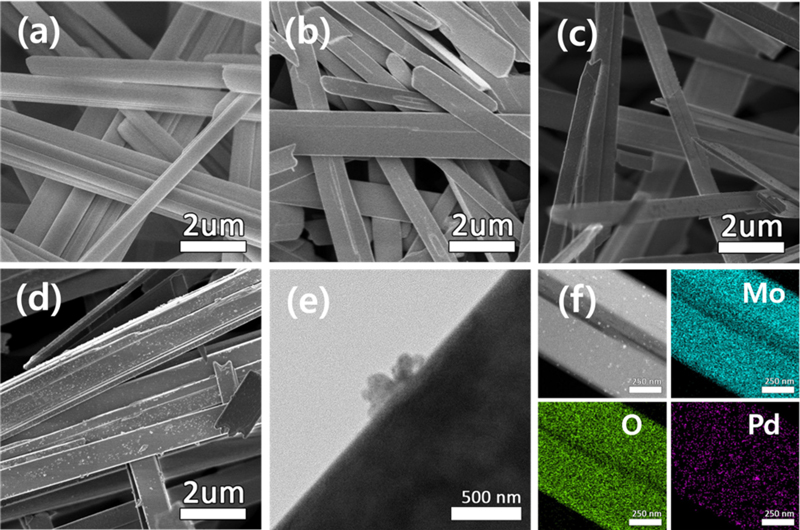
SEM images of (a) MoO3, (b) 1Pd–MoO3, (c) 3Pd–MoO3, and (d) 5Pd–MoO3 nanowires. (e) TEM image of 3Pd–MoO3. (f) Elemental mapping of 3Pd–MoO3.
Fig. 2 (e) shows a TEM image of the 3Pd–MoO3 nanowires. As evident, Pd was decorated on the surface of the MoO3 nanowires in the form of nanoparticles. The TEM elemental mapping of 3Pd–MoO3 (Fig. 2 (f)) demonstrated a uniform distribution of Pd nanoparticles (size = approximately 100 nm).
3.2 Hydrogen sensing properties of Pd–MoO3 nanowire-based colorimetric sensors
Fig. 3 illustrates the colorimetric responses of the MoO3 and Pd–MoO3 sensors to hydrogen. The sensors were exposed to 5% hydrogen for 10 min and their real-time color changes were monitored via a smartphone camera.
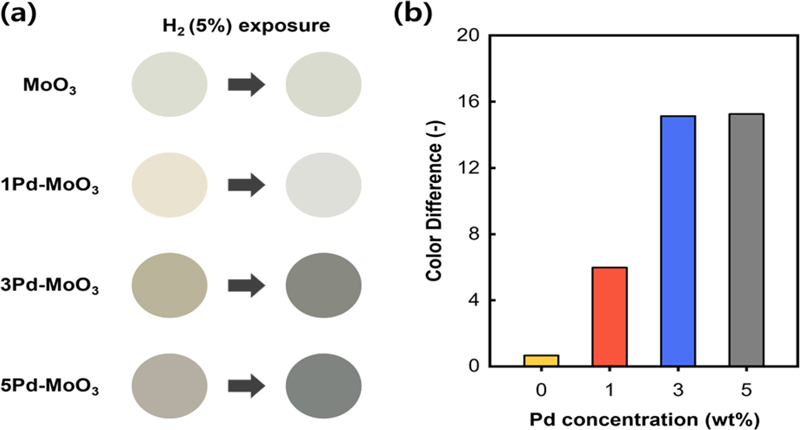
(a) Photographs of the MoO3 and Pd–MoO3 sensors before (left) and after 10 min of exposure to 5% hydrogen (right). (b) Color differences (ΔE) of the MoO3 and Pd–MoO3 sensors after 10 min of exposure to 5% hydrogen.
Fig. 3 (a) shows the color changes of the sensors after 10 min of exposure to 5% hydrogen. The pure MoO3 sensor demonstrated negligible color change; however, the Pd–MoO3 sensors exhibited significant color shifts. Specifically, all the Pd–MoO3 sensors initially had brownish colors that gradually shifted to grayish colors. These results indicate a particularly active surface reaction between Pd–MoO3 and hydrogen.
Fig. 3 (b) shows the color difference observed in the Pd–MoO3 sensors following exposure to 5% hydrogen. The ΔE values for the 1Pd–MoO3, 3Pd–MoO3, and 5Pd–MoO3 sensors were 6.00, 15.1, and 15.3, respectively. Thus, the color change plateaued when the Pd content exceeded 3 wt.%. Therefore, decorating 3 wt.% Pd is cost-effective for the detection of hydrogen with a maximum ΔE.
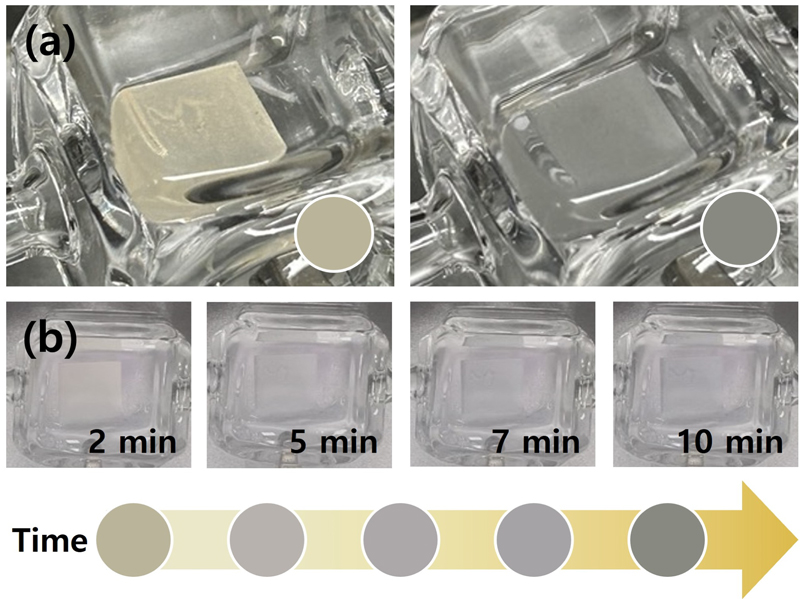
To evaluate the hydrogen detection speed of the 3Pd–MoO3 sensor, the color change was monitored at different hydrogen exposure times. The initial color of the 3Pd–MoO3 sensor was light tan (#BAB49B), which transitioned to dark gray (#888981) following exposure to 5% hydrogen for 10 min (Fig. 4 (a)). Fig. 4 (b) illustrates the progression of the color change over time. Images were captured at 2, 5, 7, and 10 min. The corresponding ΔE values were 9.04, 11.9, 13.4, and 15.1, respectively. As evident, the sensor began to exhibit a distinguishable color change (ΔE > 1.6 [13]) within 2 min. This rapid response underscores the potential of the 3Pd–MoO3 sensor for early detection of hydrogen gas leaks.
3.3 Colorimetric mechanism of Pd–MoO3 nanowires upon exposure to hydrogen
To understand the colorimetric hydrogen sensing mechanism of the 3Pd–MoO3 nanowires, XPS analysis was conducted before and after exposure to 5% hydrogen (Fig. 5). The Pd 3d XPS spectrum before hydrogen exposure revealed the presence of both Pd2+ and Pd0 peaks (Fig. 5 (a)). This indicated the partial oxidation of Pd to PdO during the synthesis process. Pd2+ peaks typically appeared at 337.4 and 342.6 eV [14] at higher binding energies compared to the Pd0 peaks (335.0 and 340.3 eV) [15]. In the Mo 3d XPS spectrum prior to hydrogen exposure, the peaks corresponding to Mo6+ were observed at 232.9 and 236.0 eV, along with smaller peaks for Mo5+ at 231.7 and 234.8 eV [10] (Fig. 5 (b)). This indicated the multivalent (Mo5+/Mo6+) nature of MoO3.
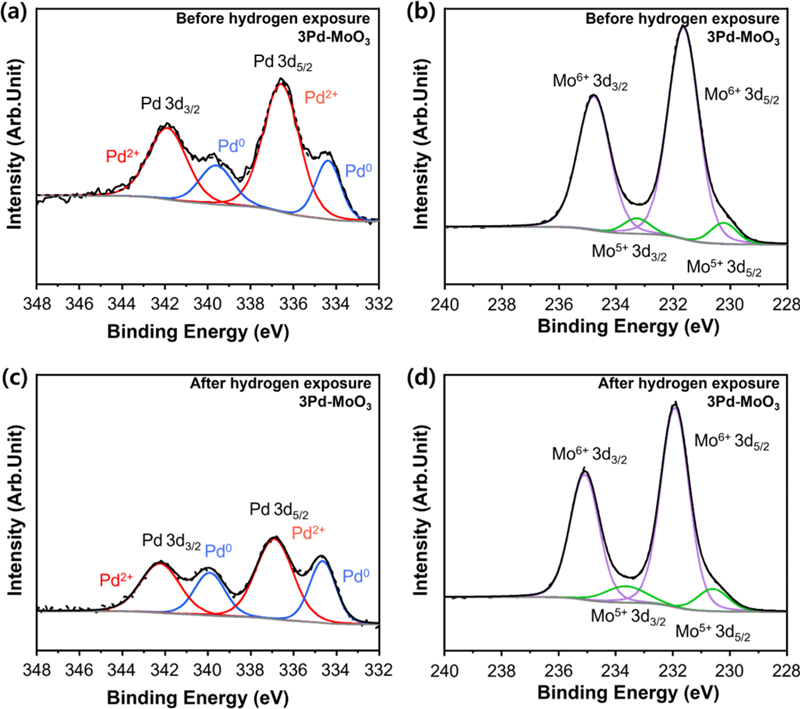
(a) XPS Pd 3d spectrum and (b) Mo 3d spectrum of the 3Pd–MoO3 before hydrogen exposure. (c) XPS Pd 3d spectrum and (d) Mo 3d spectrum of the 3Pd–MoO3 after 10 min of exposure to 5% hydrogen.
After 10 min of exposure to 5% hydrogen, the Pd and Mo 3d XPS spectra exhibited significant changes. The Pd 3d spectrum (Fig. 5 (c)) revealed an increase in the Pd0 peak intensity and decrease in the Pd2+ peaks. This implied the reduction of PdO by hydrogen. Similarly, the Mo 3d spectrum (Fig. 5 (d)) exhibited an increase in the Mo5+ peaks, implying that MoO3 was also reduced by hydrogen. The results of the quantitative analyses are presented in Table 1.

Relative fraction of Pd2+, Pd0, Mo6+, and Mo5+ in the XPS Pd and Mo 3d spectra of the 3Pd–MoO3 before and after 10 min of exposure to 5% hydrogen.
Based on XPS analysis, the colorimetric hydrogen sensing mechanism can be explained as follows [10]:
| (2) |
| (3) |
| (4) |
Upon the exposure of Pd–MoO3 to hydrogen, hydrogen molecules first adsorbed onto the Pd surface and dissociated into protons (H+) and electrons (e-) (Eq. 2). These protons and electrons were then transferred to the MoO3 surface via a spillover mechanism [16]. Subsequently, the H+ ions diffused into the MoO3, primarily interacting with the oxygen to form HxMoO3 (Eq. 3). Simultaneously, certain OH2 groups on the HxMoO3 surface detached as water molecules, thereby facilitating the formation of sub-stoichiometric MoO3-x/2 (Eq. 4). This process reduced Mo6+ to Mo5+, which corresponded to the changes observed in the Mo 3d XPS spectra (Fig. 5 (b), (d)).
During this process, the energy bandgap of MoO3 narrowed, resulting in a color change in the material. Pristine MoO3 is a semiconducting material (a band gap of 3.2 eV), wherein Mo6+ and O2- form the conduction and valence band states, respectively. The generation of Mo5+ creates new energy states within the bandgap, effectively narrowing it and causing a color change. Borgschulte et al. [17] reported that MoO3-x/2 appears blue because of intervalent charge transfer from Mo5+ to Mo6+ upon optical excitation.
However, the 3Pd–MoO3 in this study turned dark gray in hydrogen atmosphere. This suggested the influence of additional factors. This change was attributed to the colorimetric response of PdO. When exposed to hydrogen, PdO changes color owing to its reduction to Pd as per the following equation (Eq. 5). It appears dark brown at the nanoscale (100–200 nm) [18,19].
| (5) |
Notably, blue and dark brown are complementary colors; therefore, when combined, they produce a color similar to black, such as dark gray. Considering the significant PdO–to–Pd reduction observed in the XPS analysis (Fig. 5 (a), (c)), the dark gray color change resulting in the high ΔE may be attributable to the combined effects of the color shifts in both MoO3 and Pd. This finding highlights the manner in which the colorimetric properties of a material can be enhanced through the synergistic effects of the reduction of its components.
4. CONCLUSIONS
This study investigated the colorimetric hydrogen-sensing properties of MoO3 nanowires decorated with 1–5 wt.% Pd. All Pd–MoO3 nanowires exhibited a visible color change when exposed to 5% hydrogen. In particular, the MoO3 nanowires with 3 wt.% Pd (3Pd–MoO3) exhibited significant color change, with an ΔE value that exceeded 15. Furthermore, 3Pd–MoO3 demonstrated a fast response time of less than 2 min, thereby highlighting its high potential for the early detection of hydrogen gas leaks. The color shift of 3Pd–MoO3 from light brown to dark gray in the presence of hydrogen was attributable to the combined effects of the color changes in both MoO3 and Pd. With further improvements in performance, particularly in terms of the sensing speed, the 3Pd–MoO3 nanowires could be highly effective for real-time hydrogen gas leak detection in industrial settings, wherein safety and prompt intervention are essential.
Acknowledgments
This research was supported by the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (RS-2023-00239826). This work was also supported by the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ016994)” Rural Development Administration, Republic of Korea.
References
-
U. K. Zore, S. G. Yedire, N. Panadi, S. Manickam, and S. H. Sonawane, “A review on recent advances in hydrogen energy, fuel cell, biofuel and fuel refining via ultrasound process intensification”, Ultrason. Sonochem., Vol. 73, p. 105536, 2021.
[https://doi.org/10.1016/j.ultsonch.2021.105536]

-
M. L. Yue, H. Lambert, E. Pahon, R. Roche, S. Jemei, and D. Hissel, “Hydrogen energy systems: A critical review of technologies, applications, trends and challenges”, Renew. Sustain. Energy Rev., Vol. 146, p. 111180, 2021.
[https://doi.org/10.1016/j.rser.2021.111180]

-
S.-D. Han, “Review and new trends of hydrogen gas sensor technologies”, J. Sens. Sci. Technol., Vol. 19, No. 2, pp. 66-86, 2010.
[https://doi.org/10.5369/JSST.2010.19.2.067]

-
C. Wadell, S. Syrenova, and C. Langhammer, “Plasmonic Hydrogen Sensing with Nanostructured Metal Hydrides”, ACS Nano, Vol. 8, No. 12, pp. 11925-11940, 2014.
[https://doi.org/10.1021/nn505804f]

-
S. W. Kim, B. A. Trisna, M. Yin, J. Lim, T. K. Ahn, and J. Lee, “Development of a hydrogen impurity analyzer based on mobile-gas chromatography for online hydrogen fuel monitoring”, Int. J. Hydrogen Energy, Vol. 48, No. 35, pp. 13012-13023, 2023.
[https://doi.org/10.1016/j.ijhydene.2022.12.233]

-
S.-K. Kim, B.-H. Yu, and J.-W. Yoon, “NH3 sensing properties of porous CuBr films by spin-coating”, J. Sens. Sci. Technol., Vol. 30, No. 6, pp. 451-455, 2021.
[https://doi.org/10.46670/JSST.2021.30.6.451]

-
J. Lee, H. Koo, S. Y. Kim, S. J. Kim, and W. Lee, “Electrostatic spray deposition of chemochromic WO3-Pd sensor for hydrogen leakage detection at room temperature”, Sens. Actuators B Chem., Vol. 327, p. 128930, 2020.
[https://doi.org/10.1016/j.snb.2020.128930]

-
A. F. Girao and A. Completp, “Eye-readable sensors for intuitive hydrogen monitoring”, Int. J. Hydrogen Energy, Vol. 65, pp. 593-605, 2024.
[https://doi.org/10.1016/j.ijhydene.2024.04.014]

-
Z. Lv, R. Zeng, L. Zhu, Z. Qiu, M. Li, and D. Tang, “Photoelectrochemical immunoassay for prostate-specific antigen based on gasochromic reaction of Pd-MoO3”, Sens. Actuators B Chem., Vol. 370, p. 132440, 2022.
[https://doi.org/10.1016/j.snb.2022.132440]

-
X. Sun, L. Hao, L. Chen, X. Guo, C. Han, J. Chen, W. Jiao, R. Wang, and X. He, “Spray deposition of colorimetric H2 detector with Pd/MoO3 nanocomposites for rapid hydrogen leakage monitoring at room temperature”, Appl. Surf. Sci., Vol. 599, No. 15, p. 153878, 2022.
[https://doi.org/10.1016/j.apsusc.2022.153878]

-
Y. K. Kim, S.-H Hwang, S. M. Jeong, K. Y. Son, and S. K. Lim, “Colorimetric hydrogen gas sensor based on PdO/metal oxides hybrid nanoparticles”, Talanta, Vol. 188, pp. 356-364, 2018.
[https://doi.org/10.1016/j.talanta.2018.06.010]

-
S. H. Hong, Y. K. Kim, S.-H. Hwang, H.-J. Seo, and S. K. Lim, “Effect of morphology of ZnO on colorimetric hydrogen sensitivity of PdO@ZnO hybrids”, Int. J. Hydrogen Energy, Vol. 57, pp. 717-726, 2024.
[https://doi.org/10.1016/j.ijhydene.2024.01.087]

-
S. Ishikawa-Nagai, A. Yoshida, M. Sakai, J. Kristiansen, and J. D. Da Silva, “Clinical evaluation of perceptibility of color differences between natural teeth and all-ceramic crowns”, J. Dent., Vol. 37, pp. E57-E63, 2009.
[https://doi.org/10.1016/j.jdent.2009.04.004]

-
Z. Wang, Y. Zheng, J. Feng, W. Zhang, and Q. Gao, “Promoting Amination of Furfural to Furfurylamine by Metal-Support Interactions on Pd/MoO3-x Catalysts”, Chem. Eur. J., Vol. 29, No. 47, p. e202300947, 2023.
[https://doi.org/10.1002/chem.202300947]

-
X. Zhou, H.-Y. Zhou, T.-Y. Cheang, Z. W. Zhao, C.-C. Shen, K. Liang, Y.-N. Liu, Z.-K. Yang, M. Imran, and A.- W. Xu, “Monodisperse Pd Nanotetrahedrons on Ultrathin MoO3-x Nanosheets as Excellent Heterogeneous Catalyst for Chemoselective Hydrogenation Reactions”, J. Phys. Chem. C, Vol. 121, No. 49, pp. 27528-27534, 2017.
[https://doi.org/10.1021/acs.jpcc.7b10279]

-
L. Chen, A. C. Coopwe, G. P. Pez, and H. Cheng, “On the Mechanisms of Hydrogen Spillover in MoO3”, J. Phys. Chem. C, Vol. 112, No. 6, pp. 1755-1758, 2008.
[https://doi.org/10.1021/jp7119137]

-
A. Borgschulte, O. Sambalova, R. Delmelle, S. Jenatsch, R. Hany, and F. Nüesch, “Hydrogen reduction of molybdenum oxide at room temperature”, Sci. Rep., Vol. 7, No. 1, p. 40761, 2017.
[https://doi.org/10.1038/srep40761]

-
J. Kadam, S. Madiwale, B. Bashte, S. Dindorkar, P. Dhawal, and P. More, “Green mediated synthesis of palladium nanoparticles using aqueous leaf extract of Gymnema sylvestre for catalytic reduction of Cr (VI)”, SN Appl. Sci., Vol. 2, No. 11, pp. 1-13, 2020.
[https://doi.org/10.1007/s42452-020-03663-5]

-
M. R. Shaik, Z. J. Q. Ali, M. Khan, M. Kuniyil, M. E. Assal, H. Z. Alkhathlan, A. Al-Warthan, M. R. H. Siddiqui, M. Khan, and S. F. Adil, “Green Synthesis and Characterization of Palladium Nanoparticles Using Origanum vulgare L. Extract and Their Catalytic Activity”, Molecules, Vol. 22, No. 1, p. 165, 2017.
[https://doi.org/10.3390/molecules22010165]