
Mini-review on VO2-based Sensors Utilizing Metal-insulator Transition
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
With the advent of artificial intelligence and Internet of Things, demands for high-performance sensors with high sensitivity and ultrafast response for big data acquisition and processing have increased. VO2, a strongly correlated material, has been shown to exhibit a reversible and abrupt resistance change in the sub-nanosecond scale through a phase transition from an insulating to a metallic state at 68°C. The metal–insulator transition (MIT) of VO2 provides the potential for the development of highly sensitive and ultrafast high-performance sensors. This is because it can be triggered by various external stimuli, such as heat, light, gas adsorption/desorption, and strain. Therefore, attempts have been made to develop high-performance sensors by controlling the MIT of VO2 in response to external stimuli. This study reviewed recent progress in various VO2-based sensors that utilize MIT, including photodetectors, gas sensors, and strain sensors. This review is expected to serve as an overview of the approaches for controlling the MIT behavior of VO2 and provide insights into the design of high-performance sensors that exploit MIT.
Keywords:
Vanadium dioxide, Metal-insulator transition, Photodetector, Gas sensor, Strain sensor1. INTRODUCTION
Strongly correlated materials exhibit physical properties such as metal–to–insulator transition (MIT), colossal magnetoresistance, and superconductivity owing to electron-electron interactions [1,2]. Understanding the remarkable physical properties of strongly correlated materials is among the most challenging topics in condensed matter physics, offering potential solutions to overcome the limitations of conventional electronic devices [3-5]. Among the strongly correlated materials, VO2 is particularly intriguing as it exhibits a characteristic MIT behavior at a relatively near-room temperature (68°C). Further, coupled with its fast and reversible phase transition, it is known to demonstrate drastic changes in its optical, thermal, electrical, and magnetic properties [1,6-8]. By utilizing the MIT behavior of VO2, high-performance electronic devices that surpass existing limitations in conventional Si-based electronics, such as performance degradation, heat dissipation, and power consumption with high integration densities, can be fabricated. Therefore, VO2 is a promising material for applications in ultrafast-switching electronic devices. Further, the MIT behavior of VO2 is highly sensitive to external stimuli (e.g., heat, light illumination, and gas adsorption/desorption), which renders it a fascinating candidate for next-generation sensor materials [9]. Therefore, significant efforts have been devoted to exploit the MIT behavior of VO2 to develop high-performance sensors [10-15].
This review examined recent developments and progress in VO2-based sensors that utilize MIT. First, the underlying mechanism of the MIT in VO2 and its advantages in sensor applications were investigated. In the following section, we introduce approaches that can induce the MIT behavior in VO2 using external fields (light, gas adsorption/desorption, and strain) and their applications in various sensors, including photodetectors, gas sensors, and strain sensors. This review provides readers with a comprehensive overview of VO2-based sensors utilizing MIT and is expected to contribute to the development of next-generation high-performance gas sensors.
2. MECHANISM OF MIT IN VO2 AND ITS ADVANTAGES
The phase transition mechanism of a VO2 system is a topic of considerable discussion in condensed matter physics. However, there remains a lack of a definitive explanation. The phase transition in the VO2 system is generally believed to involve a complex interplay of Peierls- and Mott-transition theories [7].
Peierls theory postulates that a one-dimensional (1D) conductor is composed of atoms arranged with a uniform lattice constant, each possessing a conduction electron [16]. At lower temperatures, the system undergoes lattice distortion in pairs of atoms to achieve a more stable energy state. This distortion induces irregular spacing between atoms, thereby causing pairs of atoms to move closer or farther apart. This process is known as “dimerization.” Consequently, the altered periodicity of the chain affects the electron wave functions, creating a bandgap in the energy structure. If the modulation wave vector q=2kF (where kF is the Fermi wave vector), the lattice periodicity is doubled, resulting in an overlap of the Fermi surface and the boundary of the Brillouin zone. The critical point is that the lattice distortion causes the Brillouin zone to change. Consequently, as the Fermi surface and Brillouin zone boundary overlap, a bandgap forms in the energy band structure. With the emergence of this energy gap emerges, electrons can no longer easily transition to the conduction band. In particular, when the Fermi level, which was previously near the conduction band, is positioned within the bandgap, electrons cannot transition to the conduction band even with thermal energy. Consequently, the conductivity decreases and a transition to an insulating state is observed. In essence, Peierls’ theory describes the MIT caused by lattice distortions [17]. However, the MIT in VO2 cannot be fully explained by the Peierls mechanism alone, particularly considering the 0.6 eV bandgap in the VO2 (M1) phase and the existence of the VO2 (M2) phase.
In 1949, Mott predicted based on the band theory that the band structure changed during the MIT of VO2 [18,19]. The Mott theory suggests that electrons tend to repel each other because of Coulomb interactions. Further, as this interaction intensifies, the movement of electrons becomes restricted. Consequently, there is a sharp decline in the electrical conductivity, thus transitioning the material from a conductor to an insulator. Such an insulator is termed a Mott insulator, wherein despite a partially filled conduction band, electron movement is inhibited owing to electron-electron interactions. Thus, MIT can be modulated through doping or strain, which also provides an explanation for the existence of the VO2 (M2) phase. This phenomenon suggests that the cause of the MIT is not solely lattice distortion via the Peierls transition. Therefore, MIT can occur even in the absence of a Peierls transition.
The MIT characteristics of VO2 causes it to exhibit a high on/off ratio, high responsivity, and detectivity, coupled with the advantages of being reversible and reproducible [7-9]. The electron-electron interaction and electron-lattice interaction can be affected by various stimuli, resulting in the modulation of MIT behavior of VO2. By designing systems that can affect the electronic structure of VO2 through functionalization, high-performance sensors that respond to desired external fields can be fabricated. Consequently, the MIT behavior of VO2 must be comprehensively understood to facilitate the development of high-performance VO2-based sensors.
3. VO2-BASED SENSORS UTILIZING MIT
3.1 Photodetectors
VO2, with an energy bandgap of 0.7 eV, is a promising material for photodetectors owing to its ability to convert light from various wavelengths into electrical signals [7]. In addition, the MIT in VO2 occurs at a sub-nanosecond timescale, enabling the development of high-performance ultrafast photodetectors. However, there are several challenges in applying VO2 in photodetectors. It is attributed to the short lifetime of the photogenerated carriers and their insufficient concentration to trigger MIT [20,21]. To address these challenges, recent studies have reported the implementation of high-performance photodetectors employing heterojunctions and other functionalization approaches [10-12].
Hong et al. [10] studied the localized surface plasmon resonance (LSPR) and MIT induced by visible-near-infrared light in Ag-decorated VO2 nanorod arrays (NRs) for applications in broadband photodetectors. As shown in Fig. 1 (a), V2O5 NRs with a thickness of 250 nm were deposited using an e-beam evaporator at a glancing angle of 85°. This deposition angle satisfied the requirement of exceeding 80°, as reported by Sun et al. [22], to achieve a uniform and well-ordered array of nanorods. Subsequently, VO2 NRs with a diameter of 50 nm were fabricated via heat treatment in a reducing ambient. Further, Ag-decorated VO2 NRs were prepared by depositing Ag top electrodes with a spacing of 100 μm via e-beam evaporation.
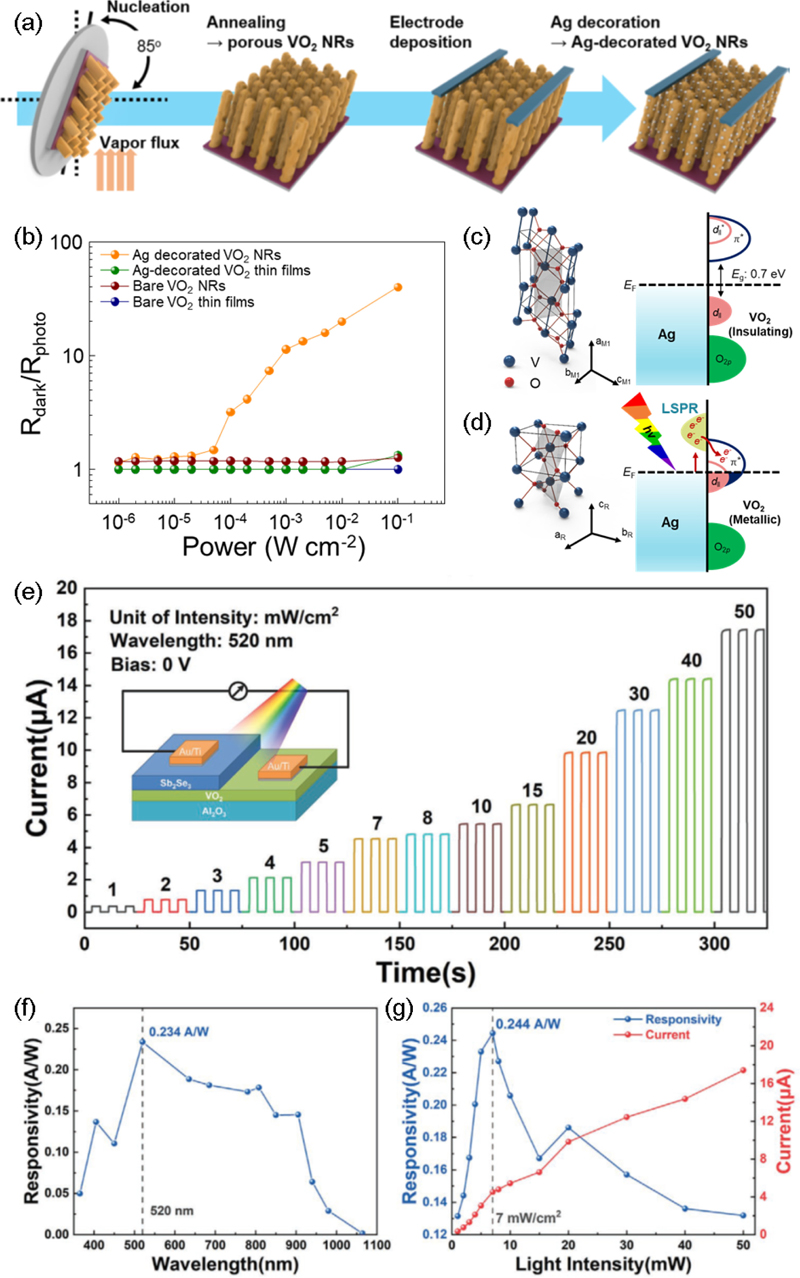
(a) Schematic of the fabrication of Ag-decorated VO2 nanorods (NRs). (b) Ratio of Rdark to Rphoto as a function of light intensity for the Ag-decorated VO2 NRs. Electronic band structure of Ag-decorated VO2 NRs (c) in the dark and (d) under illumination. Reprinted with permission from Ref. [10] Copyright (2019) American Chemical Society. (e) I–t curves of the Sb2Se3/VO2 heterostructure under different light intensities. (f) Wavelength dependent responsivities of the Sb2Se3/VO2 device. (g) Responsivities and current of the Sb2Se3/VO2 device as a function of light intensity. Reprinted with permission from Ref. [11] Copyright (2021) John Wiley and Sons.
To demonstrate that the electrostatic charging of the VO2 surface can induce MIT behavior, finite-difference time-domain (FDTD) simulations were conducted under various incident light wavelengths. The electric field distribution at the interface between the Ag NPs and the VO2 layer induced by LSPR was calculated, demonstrating a notable enhancement in the electric field at 663 nm (visible region) and 1000 nm (near-infrared region). The significant plasmon resonance frequency shift and enhanced electric field in specific regions observed in the Ag NPs support the occurrence of strong electron-electron coupling at the Ag NPs and VO2 layer interface over a broad range of incident wavelengths.
As illustrated in Fig. 1 (b), the ratio of the device resistance in the dark (Rdark) to that under white-light illumination (Rphoto) was plotted as a function of light intensity (Llight) at an applied voltage of 5 V. The Ag-decorated VO2 NRs device exhibited a slight increase in the Rdark/Rphoto ratio up to a light intensity of 50 μWcm-2. Whereas, other devices exhibited negligible Rdark/Rphoto ratios. Furthermore, as the light intensity increased up to 10 mWcm-2, the Rdark/Rphoto ratio of the Ag-decorated VO2 NRs device gradually increased, whereas the other devices maintained a negligible Rdark/Rphoto ratio below 1.3. Notably, at a white light intensity of 10 mWcm-2, the Rdark/Rphoto ratio of the Ag-decorated VO2 NRs device reached 40.1, which was comparable to the value of 47.01 induced by thermally induced MIT. This finding demonstrates that the localized heating effect owing to the plasmonic NPs induces the MIT in the Ag-decorated VO2 NRs device at light intensities exceeding 10 mWcm2.
The photo-induced MIT in the Ag-decorated VO2 NRs can be explained by the simplified band structure of VO2 near the Fermi level (Fig. 1 (c), (d)). In the dark, the Ag-decorated VO2 NRs exhibited a monoclinic VO2 (M1) phase. The formation of V–V dimers in VO2 (M1) resulted in alternating short and long V–V distances, leading to the localization of d-orbital electrons. The long distance of 3.12 Å corresponded to the separation between zigzag chains of V–V pairs, which elevated the 3d|| and 3d||* states above the Fermi energy (EF). The short distance of 2.65 Å between V–V dimers splits the nonbonding 3d|| state into the occupied 3d|| (lower energy) state and the unoccupied 3d||* (high energy) state. This resulted in the insulating behavior of VO2 (M1) with a bandgap of 0.7 eV.
When light was incident on the Ag-decorated VO2 NRs, the strong electric field generated at the Ag NPs and VO2 NRs interface owing to LSPR induced changes in the electronic states of the VO2 NRs. If this electric field is sufficiently strong to induce the MIT, a phase transition to the metallic tetragonal VO2 (R) phase occurs. In this phase, the parallel alignment of V atoms along the rutile c-axis allows all metallic V atoms to share d-orbitals and electrons, thereby forming a conduction band composed of 3d|| and 3d||* states. Consequently, the Fermi level is positioned within the overlapping region of the 3d|| and 3d||* states. This theory, combined with earlier FDTD simulation results, demonstrates that the photo-induced MIT induced by LSPR can lead to a structural phase transition (SPT) in Ag-decorated VO2 NRs across a broad range of incident wavelengths.
A heterojunction is well suited for enhancing performance metrics, such as responsivity and response time, by increasing the lifetime of charge carriers through the separation of electrons and holes. Consequently, recombination owing to the bandgap difference between the two materials is reduced. Xin et al. [11] reported a VO2/Sb2Se3 heterojunction device that maximized the charge separation efficiency and enabled the development of a self-powered photodetector (Fig. 1 (e), inset). The type-II heterojunction formed between the VO2 and Sb2Se3 facilitated efficient charge separation. When light is incident on a heterojunction, electrons in the valence band are excited to the conduction band, creating electron-hole pairs (EHPs). The built-in electric field within the heterojunction drives the separation of EHPs, with electrons migrating to Sb2Se3 and holes migrating to VO2. This separation induces a higher electric potential on the VO2 side, thereby generating a light-driven current that flows from VO2 to Sb2Se3 in the external circuit. Consequently, a high-performance self-powered photodetector is realized.
Sb2Se3 exhibits high absorption in the visible and near-infrared regions, whereas VO2, with its MIT characteristics, is responsive across a wide range of wavelengths. The VO2/Sb2Se3 heterojunction device operates over a broad wavelength range of 365–1000 nm, with a particularly outstanding responsivity at 520 nm. Fig. 1 (e) shows the transient current curves of the device under different light intensities (1–50 mWcm-2) at 520 nm. As the light intensity increased, the current magnitude continued to rise, and similarly, the responsivity increased, reaching its peak at 7 mWcm-2 (Fig. 1 (e), (f) : 0.234 A/W). Beyond this intensity, the responsivity decreased with a further increase in the light intensity. In addition, the device exhibited a fast response speed, with rise and decay times of 200 and 360 μs, respectively, as a film photodetector. The ability to generate a high photocurrent of 3 μA without an external bias further underscores the significant potential of the VO2/Sb2Se3 heterojunction device for optoelectronic applications.
Owing to the MIT characteristics of VO2 and its excellent photoresponsivity, it can be utilized in a wide range of applications, including ReRAM, smart windows, and nanoantennas. Zhou et al. [12] leveraged these properties to develop a threshold switching selector for photodetectors in the NIR (near-infrared) region and for optical neural network applications. Fig. 2 (a) and (b) depict the current-voltage characteristics of the VO2 and Au@VO2 devices at room temperature under varying light power densities from a laser (808 nm). As the power density increases, the photocurrent increases by more than two orders of magnitude, resembling the temperature-dependent I-V characteristics. This behavior is attributed to the strong IR absorption of VO2 films, which promotes temperature elevation, thereby inducing MIT in the VO2 films.
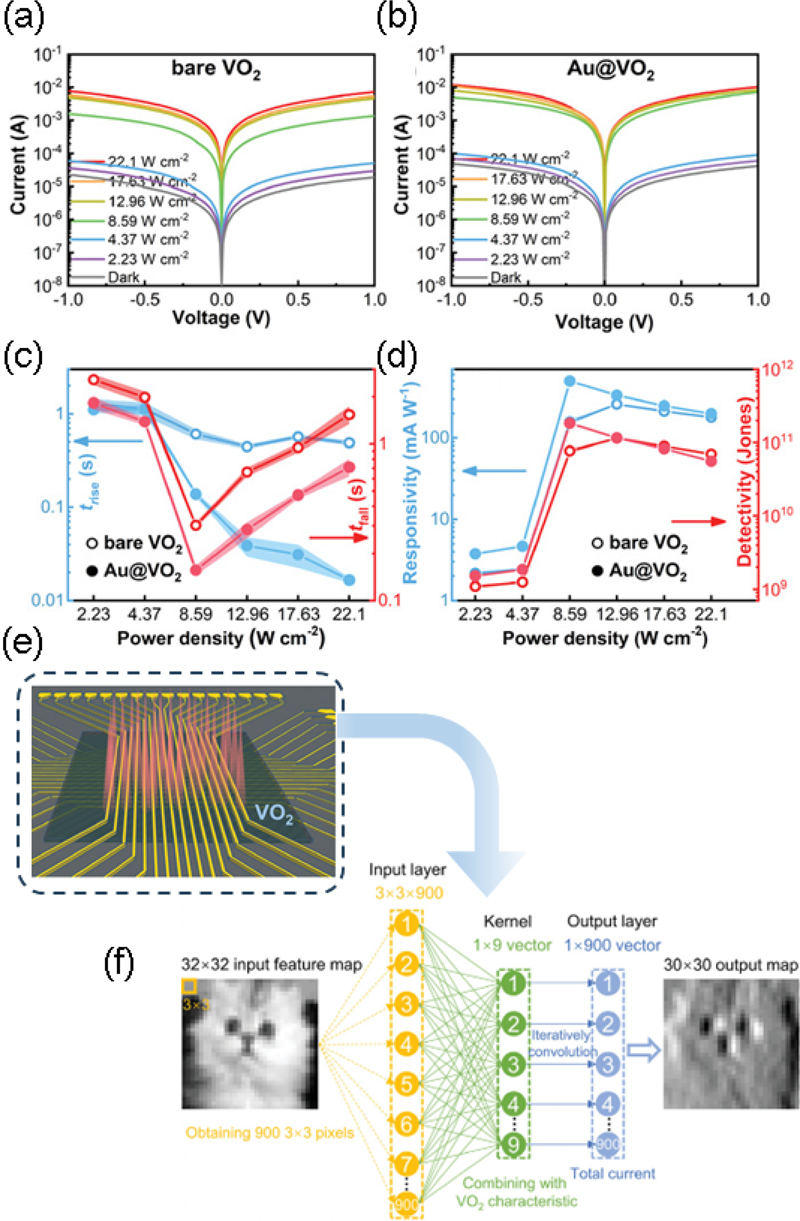
(a) I–V characteristics of (a) bare VO2 and (b) Au@VO2 photodetectors at various power densities. (c) Rise and fall times of VO2 and Au@VO2 photodetectors as a function of power densities. (d) Responsivity and detectivity of VO2 and Au@VO2 photodetectors as a function of power densities. (e) Schematic VO2 photo-selector model for the design of an optical convolution engine and (f) convolutional neural network. Reprinted with permission from Ref. [12] Copyright (2021) John Wiley and Sons.
Notably, the photocurrent of the Au@VO2 sample was saturates at 8.59 W/cm2 illumination. The resistance of the VO2 and Au@VO2 samples at 8.59 W/cm2 is comparable to their resistance under dark conditions at 70°C. Further, it is observed that bare VO2 saturates at a higher power density of 12.96 W/cm2 (Fig. 2 (c)). For the bare VO2 device, it took 446 ms to reach a stable ON state and 662 ms to switch to the OFF state. In contrast, the Au@VO2 device required only 138 ms to reach the ON state and 156 ms to switch the OFF at 8.59 W/cm2. This is attributed to n-type carrier injection by the Au nanoislands owing to LSPR-induced electron injection during the overheating process, which enhances not only the response speed but also the responsivity and detectivity of the photodetector (Fig. 2 (d)). The bare VO2 device achieves a maximum responsivity of 261.1 mA/W and a detectivity of 1.14 × 10¹¹ Jones at 12.96 W/cm2, whereas the Au@VO2 device reaches a maximum responsivity of 502.1 mA/W and a detectivity of 1.83 × 10¹¹ Jones at 8.59 W/cm2. Both devices exhibited their highest responsivity and detectivity immediately following the phase transition. However, as the power density increased further, the increase in photocurrent became negligible, and both responsivity and detectivity decreased. This indicates that the IR response is dependent on the phase transition and suggests that electron injection due to LSPR from the Au nanoislands enhances the IR response.
To explore the potential of a neural network selector, Zhou et al. [12] designed an optical convolution engine based on a VO2 device with exceptional IR response. When the cross point was irradiated with a 4 W/cm 2 laser (808 nm), it can directly switch the VO2 selector to its low-resistance state (LRS). As shown in Fig. 2 (e), although the top electrode (TE) caused a shading effect on the underlying VO2 film, the light absorption in the adjacent regions of the VO2 film, combined with Joule heating, generated sufficient thermal effects to switch the VO2 films at the electrode edges to the LRS. The significant difference between the LRS and high-resistance state (HRS), along with their integrated two-terminal structure, forms the basis for implementing an optical convolution engine. In this convolutional neural network, the current values of the VO2 device in the LRS and HRS can be used to generate binary outputs (“1” and “0”), as illustrated in Fig. 2 (e) and (f), demonstrating the computational process.
3.2 Gas sensor
While the interest in hydrogen as a renewable energy source has increased, reliable hydrogen detection has become important because of its flammability and explosiveness. It is well known that VO2 detects hydrogen well and that hydrogen adsorption can trigger the MIT in VO2 [23,24]. Therefore, several studies have focused on VO2-based high-performance gas sensors utilizing MIT [13,14,25].
Strelcov et al. [13] introduced a gas sensor based on the MIT of VO2 nanowires. The sharp phase transition characteristics of VO2 render it highly sensitive to small changes in the surrounding gas environment. To facilitate operation at room temperature, they induced phase transitions in the VO2 NWs through Joule heating by applying a current directly to the NWs. This approach eliminated the need for external heating devices because the current itself regulated the temperature within the NWs, resulting in minimal heat loss and enhanced sensor sensitivity. Moreover, the simplicity of the device design (Fig. 3 (a)) reduced response times and improved efficiency. Fig. 3 (b) illustrates the dependence of the sensor signal on the gas concentration.
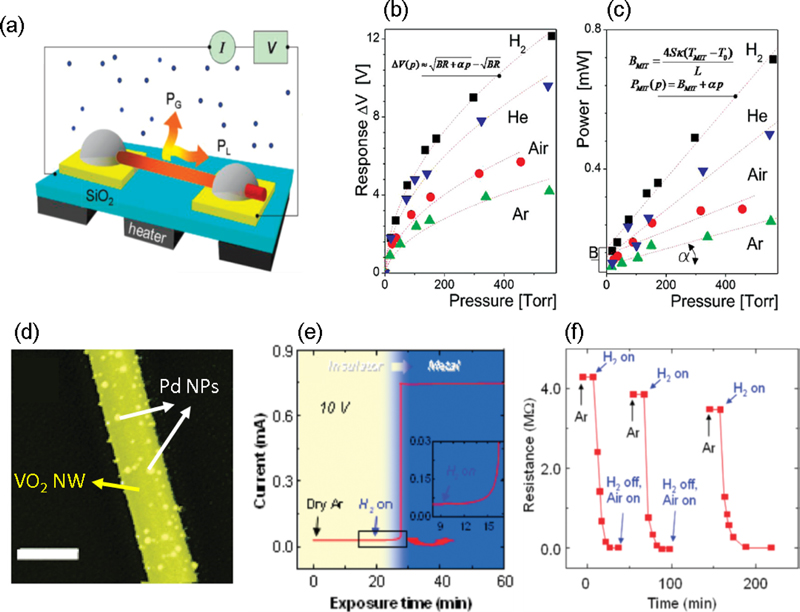
(a) Schematic of the suspended VO2 nanowire (NW) gas sensor. The voltage response of self-heated VO2 NWs to gas pulses as a function of pressure for four different gases. (c) Transition power versus gas pressure graph for self-heated VO2 nanowires. Reprinted with permission from Ref. [13] Copyright (2009) American Chemical Society. (d) SEM image of Pd-decorated VO2 NWs. (e) Current changes upon exposing Pd-decorated VO2 NWs to hydrogen. (f) Recovery rate of Pd-decorated VO2 NWs. Reprinted with permission from Ref. [14] Copyright (2009) American Chemical Society.
The sensor response, ΔV, follows a square-root dependence with the increase in concentration. In contrast, the transition power, PMIT, exhibits a linear dependence on gas pressure (Fig. 3 (c)). The operation of this mesothermistor is related to the suspended state of the NWs, as shown in Fig. 3 (a). A simple heat balance evaluation assumes that the temperature of the self-heated suspended NWs decreases linearly from the highest temperature T at the center to the electrical lead temperature T0 (T0: contact temperature). The Joule power generated in the NWs is dissipated through heat transfer to the ambient gas, heat conduction to the metal contacts, and negligible radiative losses. The suspended VO2 NW sensor operates over a wide pressure range and exhibits excellent sensitivity to both inert and reactive gases. Thus, a novel type of nanoscale gas sensor was proposed, utilizing the pressure-dependent MIT in single-crystal suspended VO2 NWs as the sensor signal. The sensor operated over a wide pressure range and offered excellent sensitivity to both inert and reactive gases. The performance of this sensor could be further enhanced by reducing the diameter of the NWs, thereby increasing their length.
Baik et al. [14] proposed a Pd-decorated VO2 nanowire device capable of highly sensitive hydrogen detection based on the MIT utilizing Pd nanoparticles (NPs) (Fig. 3 (d)). Pd NPs are considered promising as efficient catalysts for reactions with hydrogen because of their exceptional abilities to absorb and release hydrogen. A Pd-decorated VO2 nanowire device demonstrated high sensitivity specifically to hydrogen, suggesting its potential for selective hydrogen sensing applications.
As shown in Fig. 3 (e), when hydrogen was introduced into the Pd-decorated VO2 nanowire biased at 10 V, the current gradually increased. Depending on the H2 exposure time, at ambient temperatures within the range of 45–55°C, the current increased by approximately 1000-fold, indicating a transition from the insulating to the metallic state, occurring approximately 10 minutes after hydrogen exposure. At 60°C, this transition occurred within approximately 5 min. In contrast, when a hydrogen flow was introduced to the bare VO2 NWs, the change in current was less than 10%. This significant difference was attributed to the catalytic dissociation of diatomic hydrogen on the Pd NPs, which generated a substantial number of hydrogen atoms on the nanowire surface. These hydrogen atoms then “spilled over” and diffuse into the vanadium oxide, triggering MIT in the VO2 nanowires. Following the termination of hydrogen exposure after 60 min, the recovery of the nanowire conductance to its initial value was exceedingly slow. Even after 24 h in a vacuum environment (<10-5 Torr, room temperature), minimal change in the conductance value was observed. When air was introduced into the chamber, the device current began to decrease immediately, likely owing to the catalytic oxidation of hydrogen by Pd, resulting in the formation of water. However, the return of the current to its initial value required more than 4 h at 50°C, 30 min at 80°C, and 5 min at 100°C (Fig. 3 (f)).
The electrical conductance of VO2 NWs changes over two distinct timescales. The first is a rapid process occurring within 1–5 min, depending on the ambient temperature, and the second is a slower process, wherein the conductance reaches a steady state approximately 18 h after hydrogen exposure under a 1 V bias. However, when a Pd coverage corresponding to a mass thickness of 1 nm was applied, the reaction time was reduced from minutes to subseconds. This acceleration was attributed to the increased number of atomic hydrogen sources on the nanowire surface, which increased the rate of atomic H incorporation into the VO2 surface. The incorporation of atomic H into VO2 altered the electron-phonon interactions or contributes electrons, thereby increasing the electron density of VO2. These changes reduced the MIT temperature of VO2 by approximately 10°C, which is a crucial mechanism that significantly enhances the sensitivity of the hydrogen detection sensor. Researchers have proposed the possibility of electron-phonon interactions and changes in the electronic structure owing to hydrogen doping, stemming from the interaction between Pd NPs and VO2 NWs.
Li et al. [25] reported a novel hydrogen sensor based on a Pd/a-WO3/VO2 structure. Fig. 4 (a) shows a schematic of the a-WO3/VO2 hydrogen-sensor device. Following the deposition of the VO2 epitaxial layer on an aluminum oxide substrate using molecular beam epitaxy (MBE) technology, amorphous WO3 and ultrathin Pd layers were grown using magnetron sputtering and e-beam evaporation, respectively.
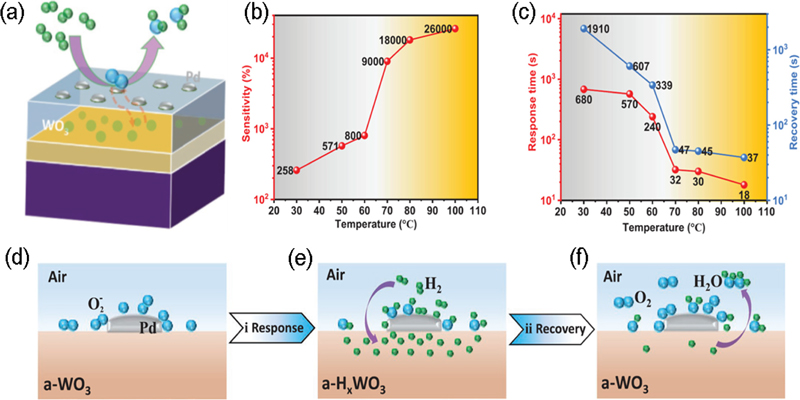
(a) Schematic of a hydrogen sensor with a heterostructure of Pd decorated a-WO3/VO2 thin film. (b) Sensitivity of Pd decorated a-WO3/VO2 heterostructure as a function of temperature. (c) Temperature dependent response and recovery time of a Pd decorated a-WO3/VO2 gas sensor. Schematic for the operating mechanism of the Pd decorated a-WO3/VO2 heterostructure in (d) pristine state, (e) response state, and (f) recovery state. Reprinted with permission from Ref. [25] Copyright (2022) John Wiley and Sons.
To investigate the effect of the VO2 phase transition on the performance of the sensor, the sensitivity was measured following its exposure to hydrogen gas (5 ppm) at 30°C and 80°C. The sensitivities in the insulating state (30°C) and the metallic state (80°C) were 258% and 18,000%, respectively, indicating a sharp increase in sensitivity following the phase transition (Fig. 4 (b)). In addition, the response and recovery times were significantly reduced to 680 and 30 s at 30°C, and 1910 and 45 s at 80°C, respectively (Fig. 4 (c)). These results suggest that the phase transition properties of VO2 have a substantial impact on the performance of the sensor.
The entire sensing process of the Pd/a-WO3/VO2 sensor involves the following steps (Fig. 4 (d–f)):
| (1) |
| (2) |
| (3) |
In the pristine state (Fig. 4 (d)), oxygen adsorbed on the surface is reduced to O2- ions owing to the catalytic effect of Pd NPs and interfacial electron transfer within the a-WO3/VO2 layer. When the sensor is exposed to H2 gas, in the response state (Fig. 4 (e)), Pd NPs catalytically dissociate hydrogen into H+ ions, which are then drawn into the WO3 layer via an electron–proton co-doping process at the interface. This results in the metallization of the H-doped WO3 layer. This process decreases the resistance of the a-WO3 layer, and the sensing conductance signal is synchronously detected. In the third stage (Fig. 4 (f)), synthetic air is introduced to restore the device to its initial state. Here, the Pd NPs catalyze the dissociation of oxygen molecules into O2- ions, which react with H atoms released from the a-WO3 layer to form H2O, subsequently evaporating from the surface owing to external heating and the intrinsic exothermic reaction. The excess electrons generated in the reaction are transferred back to the Pd NPs, where they reform O2- ions. During this process, the phase transition from VO2 (M) to VO2 (R) facilitates the transfer of more number of electrons from VO2 to the WO3 layer, facilitating the conductive HxWO3 formation and promoting dehydrogenation during the recovery process. The authors demonstrated significant performance enhancements, including detection limits, response time, and sensitivity, through interfacial charge transfer, depending on the electronic phase of VO2. These results provide valuable insight into the design of high-performance gas sensors utilizing the MIT of VO2.
3.3 Strain sensor
VO2 is an exceptionally advantageous material for mimicking human body functions or detecting dynamic changes owing to its high sensitivity, rapid response, and metal-insulator transition (MIT) characteristics [26]. In addition, repeated strain can induce phase transitions between the M1 and M2 phases within VO2 [15]. These properties render VO2 a promising candidate for the fabrication of high-performance strain sensors.
Hu et al. [15] introduced a flexible strain sensor that induced MIT through an external strain in a VO2 nanobeam structure. As shown in Fig. 5 (a), tensile and compressive strains were applied incrementally in steps of 0.05%, and the corresponding changes in the I–V characteristics were observed. In contrast to piezotronic-based strain sensors, the I–V curve of the VO2 nanobeam device exhibited downward and upward shifts under tension and compression, respectively. This distinct response to different types of strain was attributed to the M1-M2 phase transition within the VO2 nanobeam.
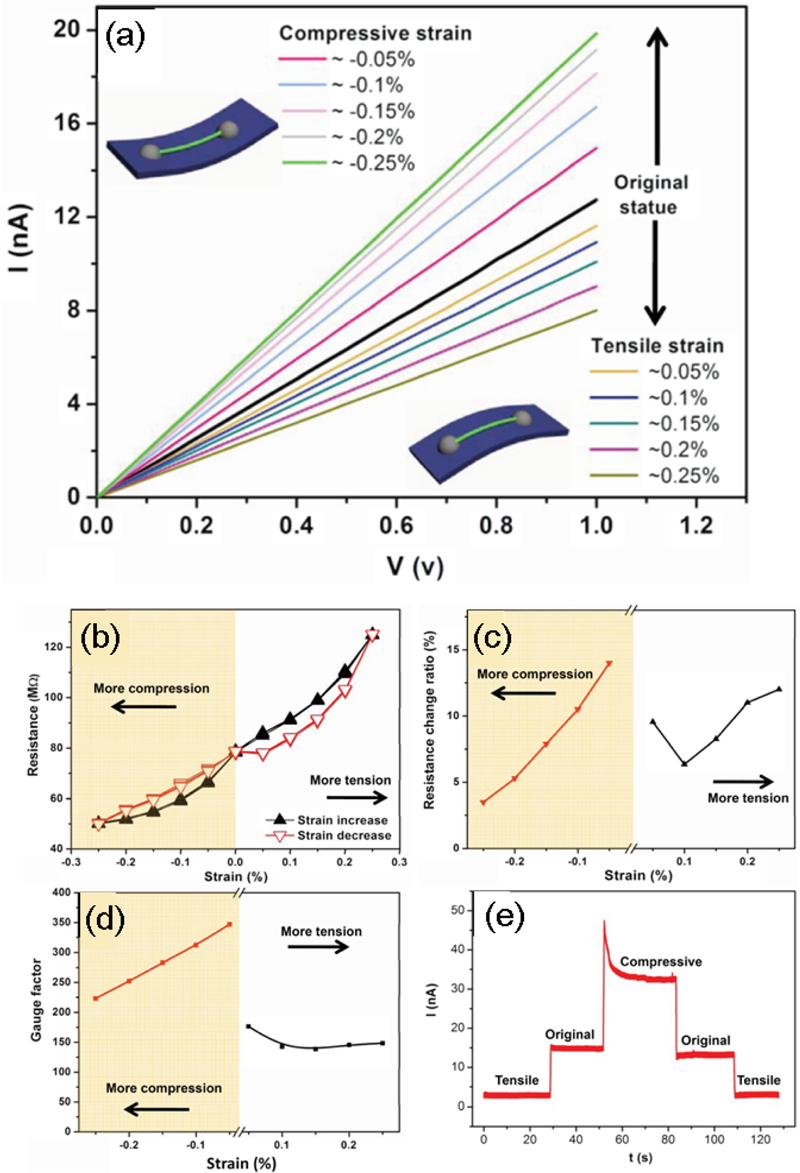
(a) I–V characteristics of VO2 nanobeam under different tensile and compressive strains. (b) Resistance hysteresis of the VO2 nanobeam under external strain and strain release. (c) Resistance changes of VO2 nanobeam owing to strain in (b). (d) Gauge factor as a function of strain derived from (b). (e) Response of VO2 nanobeam to strain switching. Reprinted with permission from Ref. [15] Copyright (2010) John Wiley and Sons.
As shown in Fig. 5 (b), the resistance changes owing to the strain increased under tension and the decreased under compression. To verify the stability of the device, the original state, tension, release, compression, and release were sequentially scanned thrice. The VO2 nanobeam exhibited a stable and reproducible performance under repeated tensile and compressive strains. In addition, the variation in the resistance with strain was measured to confirm the operating principle of the device (Fig. 5 (c)). As the compression strain increased, the resistance changes decreased, which was attributed to the stepwise reduction of the M2 phase induced by the pre-applied tensile strain in the localized regions of the VO2 nanobeam. As the tensile strain increased, a sufficient amount of the original M1 phase transformed into the M2 phase, which resulted in a relatively stable resistance change.
Most semiconductor-based strain mechanisms involve changes in the band structure of the semiconductor material under strain, typically leading to linear resistance changes. However, in contrast to conventional devices, a VO2 nanobeam device does not exhibit a hysteresis effect (Fig. 5 (b)) and does not perfectly match the linear relationship with the applied stress, as shown in Fig. 5 (c). Therefore, the dominant cause of the resistance change in the VO2 nanobeam device under strain is likely the phase transition rather than the piezoresistance effect.
The performance of the strain sensor is characterized by its gauge factor, with the VO2 nanobeam sensor exhibiting a maximum gauge factor of 347 (Fig. 5 (d)). In contrast to other types of sensors, the VO2 nanobeam sensor achieves its maximum gauge factor at the lowest applied stress. Although the gauge factor decreases with increasing stress, it remains higher than that of conventional metal strain gauges and doped Si strain sensors. In addition, as demonstrated in Fig. 5 (e), the response time under a 1 V bias shows a rapid response to strain switching. Because there is no significant change in the current at a 1 V bias across the original, compression, and tensile states of the VO2 nanobeam, the Joule self-heating effect can be excluded. The relatively balanced performance in both the compression and tensile states suggests that the VO2 nanobeam strain sensor is well-suited for applications in low mechanical strain ranges.
4. CONCLUSIONS
Thus, this study reviewed various approaches to controlling the MIT behavior of VO2 (a strongly correlated material) through external stimuli (e.g., light, gas adsorption/desorption, and mechanical stress), and their applications to various sensors. Although various approaches have been proposed, the physical and chemical phenomena and properties at the interfaces within VO2-based heterojunctions must be investigated. Understanding the complex interactions in multidimensional environments and their effects on the control of the MIT behavior of VO2 has significant fundamental scientific importance and can significantly contribute to the development and implementation of next-generation high-performance sensors.
Acknowledgments
This work was supported by the Korea Institute for Advancement of Technology (KIAT) grant funded by the Ministry of Education (Grant No. P0025690, Semiconductor-Specialized University), Basic Science Research Capacity Enhancement Project through Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (Grant No. 2019R1A6C1010024), and the ‘Regional Innovation Strategy (RIS)’ through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea (Grant No. 2021RIS-002).
References
-
M. Imada, A. Fujimori, and Y. Tokura, “Metal-insulator transitions”, Rev. Mod. Phys., Vol. 70, pp. 1039-1263, 1998.
[https://doi.org/10.1103/RevModPhys.70.1039]

-
E. Morosan, D. Natelson, A. H. Nevidomskyy, and Q. Si, “Strongly Correlated Materials”, Adv. Mater., Vol. 24, No. 36, pp. 4896-4923, 2012.
[https://doi.org/10.1002/adma.201202018]

-
Y. J.Lee, Y. Kim, H. Gim, K. Hong, and H. W. Jang, “Nanoelectronics Using Metal–Insulator Transition”, Adv. Mater., Vol. 36, No. 5, p. 2305353, 2024.
[https://doi.org/10.1002/adma.202305353]

-
P. Schofield, A. Bradicich, R. M. Gurrola, Y. Zhang, T. D. Brown, M. Pharr, P. J. Shamberger, and S. Banerjee, “Harnessing the Metal–Insulator Transition of VO2 in Neuromorphic Computing”, Adv. Mater., Vol. 35, No. 37, p. 2205294, 2023.
[https://doi.org/10.1002/adma.202205294]

-
J. L. Andrews, D. A. Santos, M. Meyyappan, R. S. Williams, and S. Banerjee, “Building Brain-Inspired Logic Circuits from Dynamically Switchable Transition-Metal Oxides”, Trends Chem., Vol. 1, No. 8, pp. 711-726, 2019.
[https://doi.org/10.1016/j.trechm.2019.07.005]

-
F. J. Morin, “Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature”, Phys. Rev. Lett., Vol. 3, No. 1, p. 34, 1959.
[https://doi.org/10.1103/PhysRevLett.3.34]

-
Z. Shao, X. Cao, H. Luo, and P. Jin, “Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials”, NPG Asia Mater., Vol. 10, No. 7, pp. 581-605, 2018.
[https://doi.org/10.1038/s41427-018-0061-2]

-
M. Brahlek, L. Zhang, J. Lapano, H.-T. Zhang, R. Engel-Herbert, N. Shukla, S. Datta, H. Paik, and D. G. Schlom, “Opportunities in vanadium-based strongly correlated electron systems”, MRS Commun., Vol. 7, No. 1, pp. 27-52, 2017.
[https://doi.org/10.1557/mrc.2017.2]

-
Z. Yang, C. Ko, and S. Ramanathan, “Oxide Electronics Utilizing Ultrafast Metal-Insulator Transitions”, Annu. Rev. Mater. Res., Vol. 41, No. 1, pp. 337-367, 2011.
[https://doi.org/10.1146/annurev-matsci-062910-100347]

-
K. Hong, C. W. Moon, J. M. Suh, T. H. Lee, S.-I Kim, S. Lee, and H. W. Jang, “Daylight-Induced Metal–Insulator Transition in Ag-Decorated Vanadium Dioxide Nanorod Arrays”, ACS Appl. Mater. Interfaces, Vol. 11, No. 12, pp. 11568-11578, 2019.
[https://doi.org/10.1021/acsami.8b19490]

-
Y. Xin, J. Jiang, Y. Liu, H. Liang, Y.-J. Zeng, and Z. Ye, “Self-Powered Broad Spectral Photodetector with Ultrahigh Responsivity and Fast Response Based on Sb2Se3/VO2 Heterojunction”, Adv. Mater. Interfaces, Vol. 8, No. 10, p. 2100058, 2021.
[https://doi.org/10.1002/admi.202100058]

-
X. Zhou, L. Zhao, W. Zhen, Y. Lin, C. Wang, T. Pan, L. Li, G. Du, L. Lu, X. Cao, and D. Li, “Phase-Transition-Induced VO2 Thin Film IR Photodetector and Threshold Switching Selector for Optical Neural Network Applications”, Adv. Electron. Mater., Vol. 7, No. 5, p. 2001254, 2021.
[https://doi.org/10.1002/aelm.202001254]

-
E. Strelcov, Y. Lilach, and A. Kolmakov, “Gas Sensor Based on Metal−Insulator Transition in VO2 Nanowire Thermistor”, Nano Lett., Vol. 9, No. 6, pp. 2322-2326, 2009.
[https://doi.org/10.1021/nl900676n]

-
J. M. Baik, M. H. Kim, C. Larson, C. T. Yavuz, G. D. Stucky, A. M. Wodtke, and M. Moskovits, “Pd-Sensitized Single Vanadium Oxide Nanowires: Highly Responsive Hydrogen Sensing Based on the Metal−Insulator Transition”, Nano Lett., Vol. 9, No. 12, pp. 3980-3984, 2009.
[https://doi.org/10.1021/nl902020t]

-
B. Hu, Y. Ding, W. Chen, D. Kulkarni, Y. Shen, V. V. Tsukruk, and Z. L. Wang, “External-Strain Induced Insulating Phase Transition in VO2 Nanobeam and Its Application as Flexible Strain Sensor”, Adv. Mater., Vol. 22, No. 45, pp. 5134-5139, 2010.
[https://doi.org/10.1002/adma.201002868]

-
R. Peierls, More surprises in theoretical physics, Princeton, Princeton University Press, NJ, pp. 1-106, 1991.
[https://doi.org/10.1515/9780691214320]

-
J. B. Goodenough, “The two components of the crystallographic transition in VO2”, J. Solid State Chem., Vol. 3, No. 4, pp. 490-500, 1971.
[https://doi.org/10.1016/0022-4596(71)90091-0]

-
N. F. Mott, Metal-insulator transitions, London, Taylor and Francis, UK, pp. 1-286, 2004.
[https://doi.org/10.1201/b12795]

-
N. F. Mott, “The Basis of the Electron Theory of Metals, with Special Reference to the Transition Metals”, Proc. Phys. Soc. Sect. A, Vol. 62, No. 7, p. 416, 1949.
[https://doi.org/10.1088/0370-1298/62/7/303]

-
M. M. Qazilbash, M. Brehm, B.-G. Chae, P.-C. Ho, G. O. Andreev, B.-J. Kim, S. J. Yun, A. V. Balatsky, M. B. Maple, F. Keilmann, H.-T. Kim, and D. N. Basov, “Mott Transition in VO2 Revealed by Infrared Spectroscopy and Nano-Imaging”, Science, Vol. 318, No. 5857, pp. 1750-1753, 2007.
[https://doi.org/10.1126/science.1150124]

-
M. M. Qazilbash, K. S. Burch, D. Whisler, D. Shrekenhamer, B. G. Chae, H. T. Kim, and D. N. Basov, “Correlated metallic state of vanadium dioxide”, Phys. Rev. B, Vol. 74, No. 20, p. 205118, 2006.
[https://doi.org/10.1103/PhysRevB.74.205118]

-
Y. Sun, X. Xiao, G. Xu, G. Dong, G. Chai, H. Zhang, P. Liu, H. Zhu, and Y. Zhan, “Anisotropic vanadium dioxide sculptured thin films with superior thermochromic properties”, Sci. Rep., Vol. 3, No. 1, p. 2756, 2013.
[https://doi.org/10.1038/srep02756]

-
J. Wei, H. Ji, W. Guo, A. H. Nevidomskyy, and D. Natelson, “Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams”, Nat. Nanotechnol., Vol. 7, No. 6, pp. 357-362, 2012.
[https://doi.org/10.1038/nnano.2012.70]

-
H. Yoon, M. Choi, T.-W. Lim, H. Kwon, K. Ihm, J. K. Kim, S.-Y. Choi, and J. Son, “Reversible phase modulation and hydrogen storage in multivalent VO2 epitaxial thin films”, Nat. Mater., Vol. 15, No. 10, pp. 1113-1119, 2016.
[https://doi.org/10.1038/nmat4692]

- B. Li, Z. Wang, S. Zhao, C. Hu, L. Li, M. Liu, J. Zhu, T. Zhou, G. Zhang, J. Jiang, and C. Zou, “Enhanced Pd/a-WO3/VO2 Hydrogen Gas Sensor Based on VO2 Phase Transition Layer”, Small Methods, Vol. 6, No. 12, p. 2200931, 2022.
-
M. Darwish, Y. Zhabura, and L. Pohl, “Recent Advances of VO2 in Sensors and Actuators”, Nanomaterials, Vol. 14, No. 7, p. 582, 2024.
[https://doi.org/10.3390/nano14070582]
