Recent Trends in Gas Sensors for Food Spoilage Monitoring
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Monitoring and controlling food quality during cultivation, distribution, and storage is crucial for ensuring public health and safety. The spoilage of perishable foods such as meat and fish is often accompanied by the emission of trace gases, including ammonia and hydrogen sulfide. Early detection of these gases is vital for the timely intervention and prevention of food spoilage. Metal-oxide-based gas sensors have shown considerable potential for addressing this challenge, owing to their high sensitivity and ability to detect spoilage-related gases at sub-ppb concentrations. Recent advancements in the development of metal-oxide gas sensors, particularly those operating at room temperature, have provided the foundation for practical applications in the food industry. These sensors facilitate real-time monitoring of food freshness, thus offering a novel strategy for spoilage detection and management. High-performance room-temperature gas sensors are expected to enhance the efficiency and safety of food storage solutions, thus improving overall food quality management across the supply chain.
Keywords:
Food spoilage, Monitoring, Oxide semiconductors1. INTRODUCTION
Food is an indispensable component of human life, with its quality and safety directly linked to human health. According to the World Health Organization (WHO), approximately 600 million people are affected annually by foodborne illnesses, posing a significant burden on healthcare systems and impeding social and economic development [1]. Therefore, maintaining food freshness and preventing spoilage is essential. Many researchers have explored various technologies, such as microbial sterilization of packaging using different agents with strong oxidizing properties and monitoring food freshness by detecting gases generated during spoilage [2,3]. The real-time monitoring of food quality via gas detection has garnered considerable interest [4]. Perishable foods such as meat, vegetables, and fruits emit various gases during ripening and spoilage [5]. Microbial enzymes degrade amino acids in protein-rich environments, resulting in the formation of volatile amines such as ammonia (NH3) and trimethylamine (TMA) [6]. In the case of fruits, ethylene (C2H4) is a representative gas produced by the ripening process, and vegetables and flowers also emit ethylene [7]. However, these gases are emitted at extremely low concentrations, often parts per billion (ppb), necessitating their detection. From this perspective, developing a gas sensor that can detect low concentrations of these gases would provide a significant advantage by offering real-time information to ensure the safety and freshness of food for consumption.
Food sensors have been conventionally developed using various analytical techniques such as gas chromatography–mass spectrometry and Raman spectroscopy [8-10]. Although these techniques provide high accuracy and detailed analysis, they require expensive equipment, which results in high operating costs. Furthermore, the large sizes of these instruments restrict their potential for miniaturization and user-friendliness. Although portable pH sensors based on visual spectroscopy have been reported, their effectiveness in real-time food quality monitoring is often constrained by slow response times and low sensitivities [1,11,12].
To overcome these challenges, researchers have recently proposed food-monitoring platforms using chemiresistive-type gas sensors [13,14]. Most resistive gas sensors are based on metaloxide semiconductors (Fig. 1), which offer several advantages, including low cost, high stability, potential for miniaturization, high sensitivity, and fast response and recovery times [15,16]. For example, Park et al. [17] demonstrated that trimethylamine gas, released during fish spoilage, can be detected at low concentrations with improved selectivity over other gases by creating a composite structure of p-type oxide Cr3O4 and n-type oxide SnO2.
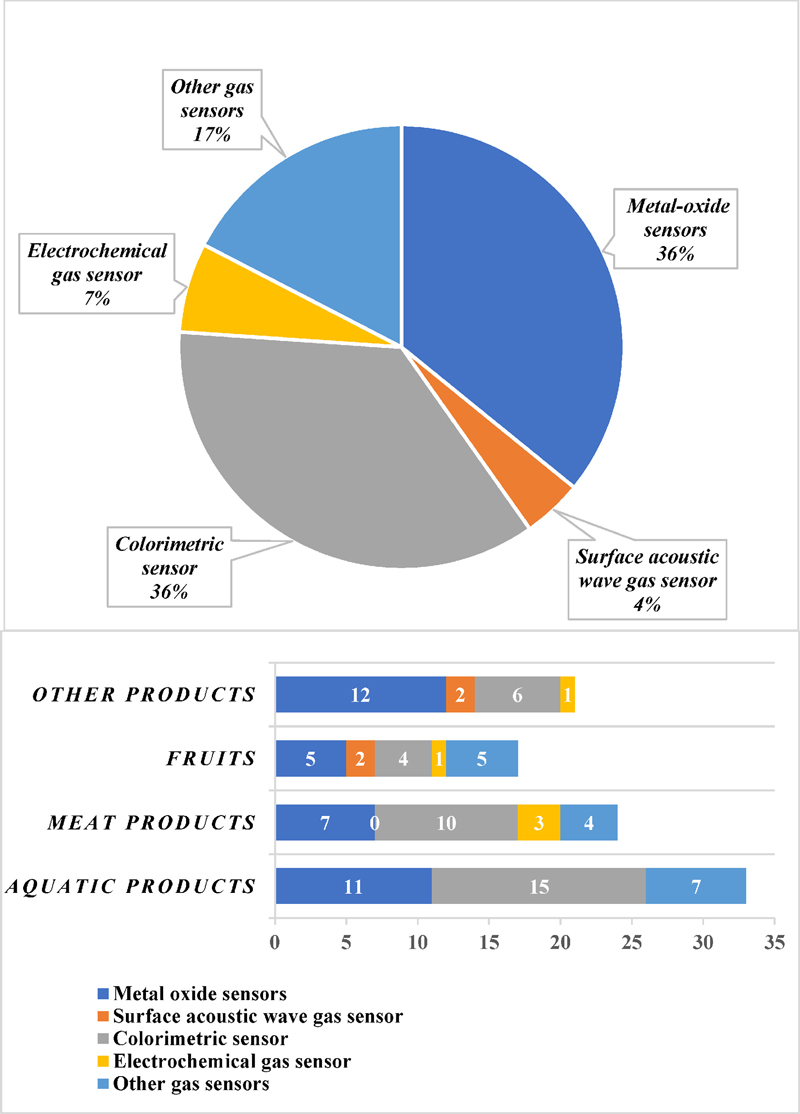
Different types of gas sensors for food freshness monitoring (upper). Distribution of gas sensors depends on the products (lower). Reprinted with permission [18] copyright (2023) MDPI.
This paper describes the mechanism of chemiresistive gas sensors. Subsequently, research trends in food spoilage and freshness monitoring based on chemiresistive gas sensors are introduced.
2. OVERVIEW OF CHEMIRESISTIVE GAS SENSORS
2.1 Sensing Mechanism of Metal Oxide-Based Chemiresistive Gas Sensors
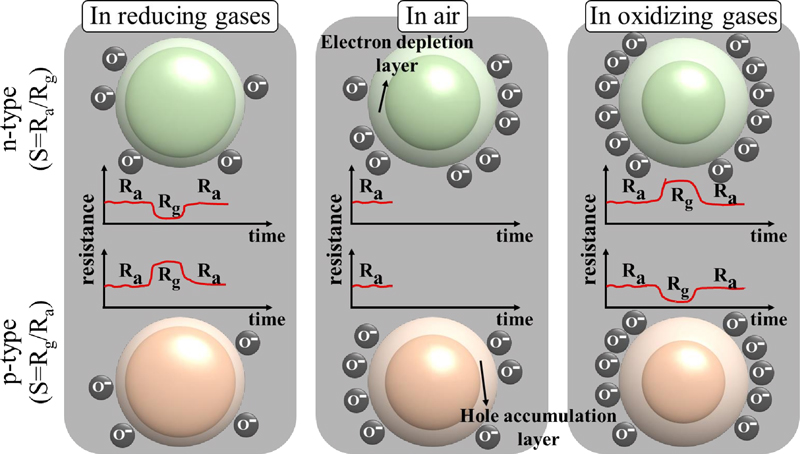
Gas sensors have been extensively studied based on a variety of sensing principles, including electrochemical, infrared absorption, and chemical resistance. In particular, chemiresistive gas sensors have garnered significant attention owing to their high sensitivity, stability, rapid response times, and low production costs. The operating principle of chemiresistive gas sensors is based on the change in electrical resistance that occurs when the sensing materials interact with the target gas molecules. Semiconductor metal oxides (SMOs), commonly used in chemiresistive gas sensors, are classified as either p-type or n-type, depending on their intrinsic properties. Gases are typically classified into two types: i) oxidizing gases (e.g., nitrogen oxide (NO2), toluene (C6H5CH3), and xylene (C8H10)) and ii) reducing gases (e.g., hydrogen sulfide (H2S), ammonia, ethanol (C2H6O), and ethylene). Oxidizing gases are known to capture electrons from the sensing materials upon interaction, whereas reducing gases generally donate electrons to the sensing material. Consequently, the resistance of the sensing materials varies depending on whether they are exposed to air (oxygen) or the target gases.
For example, in the case of n-type SMOs, the surface reacts with atmospheric oxygen, decreasing the number of electrons, which leads to the formation of an “electron depletion region.” During this process, the surface becomes negatively charged with species such as O2-, O-, and O2-. When n-type SMOs are exposed to oxidizing gases, the gas molecules extract electrons from the sensing materials, thereby expanding the depletion region and increasing the resistance (Fig. 2). Conversely, p-type SMOs, predominantly composed of holes, lose electrons by reacting with oxygen, forming a hole-accumulation region. When p-type SMOs react with oxidizing gases, the hole accumulation region becomes larger owing to the decreased number of electrons. In both cases, changes in the electron depletion region or hole accumulation region lead to variations in the resistance. This resistance change is quantified in terms of the response sensitivity (S = Rg/Ra or Ra/Rg, where Rg is the resistance in the presence of gas and Ra is the resistance in ambient air). Owing to their high sensitivity to resistance changes, SMO-based gas sensors are particularly advantageous for miniaturization as semiconductor gas sensors. Thus, SMO-based gas sensors are promising candidates for monitoring systems designed to detect food spoilage.
3. GAS SENSORS FOR FOODS MONITORING
3.1 Monitoring of Meat and Fish Spoilage
Meat and fish are consumed globally because of their rich flavor and high nutritional value. However, their high protein content makes them susceptible to spoilage because microbes quickly break down proteins into amino acids, leading to the production of various biogenic and volatile amine compounds. The most common volatile amines are ammonia, trimethylamine, and dimethylamine (DMA), which degrade food quality and are harmful to human health. These volatile amine compounds can act as key indicators for assessing the freshness of meat and fish [19].
Ammonia is primarily produced through the metabolic activities of bacteria and fungi [20]. Considering ammonia is produced and released when meat spoils, its detection may play a crucial role in monitoring food freshness and determining the degree of spoilage. To date, research on SMO-based gas sensors has predominantly focused on high-temperature operations to achieve high reactivity. However, this approach hinders the feasibility of real-time food freshness monitoring owing to its high power consumption, degradation of materials, and potential contamination or spoilage of food [21]. Sonwal et al. [3] reported a real-time meat-freshness monitoring system at room temperature based on the synergistic effect of sulfur-rich WS2 and rGO nanoflakes (Fig. 3 (a)). First, sulfur-rich WS2 was synthesized using oxalic acid, which facilitated the uniform and homogenous formation of WS2. The uniformity of the sulfur-rich WS2 improved its sensing performance (Fig. 3 (b)). Additionally, the sulfur-rich WS2 decorated rGO exhibited superior performance with the creation of p-p junctions (Fig. 3 (c)), highlighting the crucial role of sulfur in improving sensor response and that the heterojunction with rGO further improved the gas reactivity by increasing the surface area. The magnitude of the resistance signal increases proportionally with the degree of meat spoilage, demonstrating the potential of this sensor for food safety monitoring.
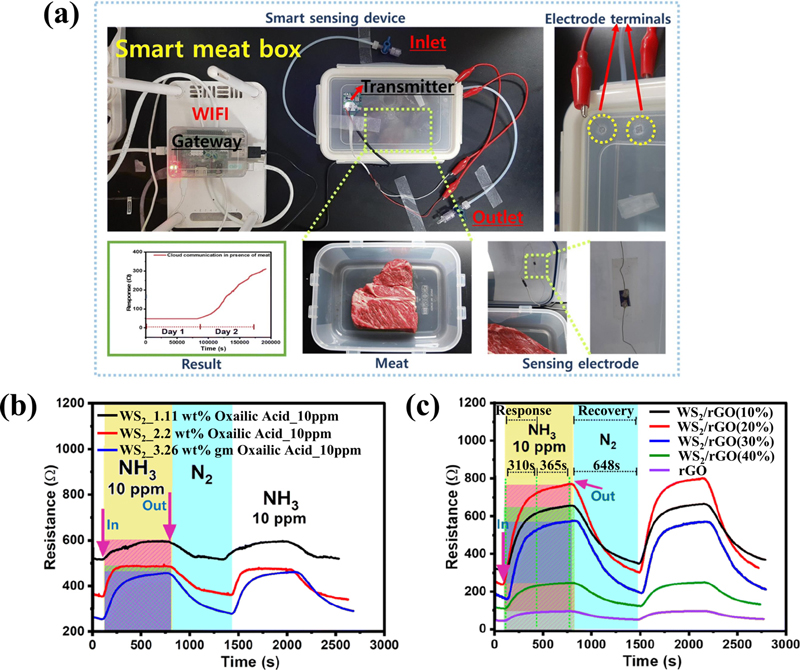
(a) Schematic representation of the real-time meat spoilage monitoring setup. (b, c) Chemiresistive response optimization of sulfur-rich WS2 and WS2/rGO to 10 ppm NH3. Reprinted with permission [3] Copyright 2024, The Royal Society of Chemistry.
Similarly, Li et al. [22] developed ammonia gas sensors with long-term stability and a low limit of detection using an Fe2Mo3O8/MoO2@MoS2 nanocomposite, which showed excellent selectivity for interfering gases such as hydrogen sulfide, nitrogen dioxide, methanol (CHOH), carbon oxide (CO), and ethanol while maintaining a nearly constant response without degradation for over four weeks (Fig. 4). This superior performance was attributed to the heterojunction interface, which enhanced electron transfer. In addition, the bonding of Fe2Mo3O8, characterized by a low free energy change (ΔG) during the oxidation process, helps promote NH3 adsorption and catalytic oxidation even at room temperature.
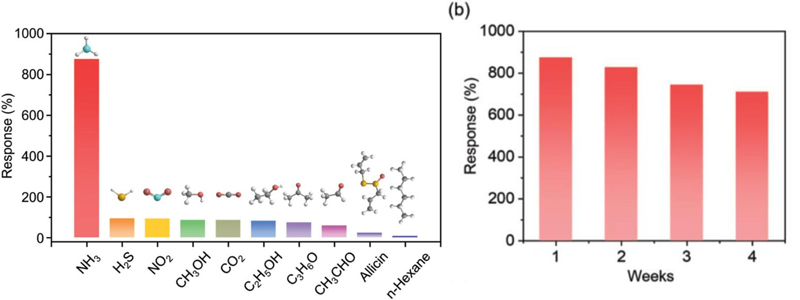
Responses of the Fe2Mo3O8/MoO2@MoS2-900°C sensor to 30 ppm of NH3 and interference gases at room temperature and 5% RH and long-term stability of Fe2Mo3O8/MoO2@MoS2-900°C sensor. Reprinted with permission [22] Copyright 2024 WILEY.
Although extensive research has been conducted on ammonia gas detection using chemiresistive gas sensors [23-26], studies specifically focusing on ammonia gas sensors for meat-freshness monitoring are still in the early stages and require further investigation.
Trimethylamine, a volatile amine, has an unpleasant odor similar to that of ammonia that becomes particularly prominent when fish spoil, as large quantities of trimethylamine are produced during the reduction of trimethylamine oxide (TMAO) in fish tissue. Therefore, trimethylamine can be used as an indicator to monitor environmental pollution and control the freshness of fish. Li et al. [27] reported a dual-function gas sensor capable of selectively detecting hydrogen sulfide and trimethylamine at different temperatures by fabricating MoO3 nanobelt thin films with an average length of 200 μm using drop coating (Fig. 5). Selective and sensitive detection was achieved through an acid–base interaction between acidic MoO3 and basic trimethylamine. Although similar studies focusing on high sensitivity have been intensively conducted, room-temperature gas sensors have also been investigated [17,28].
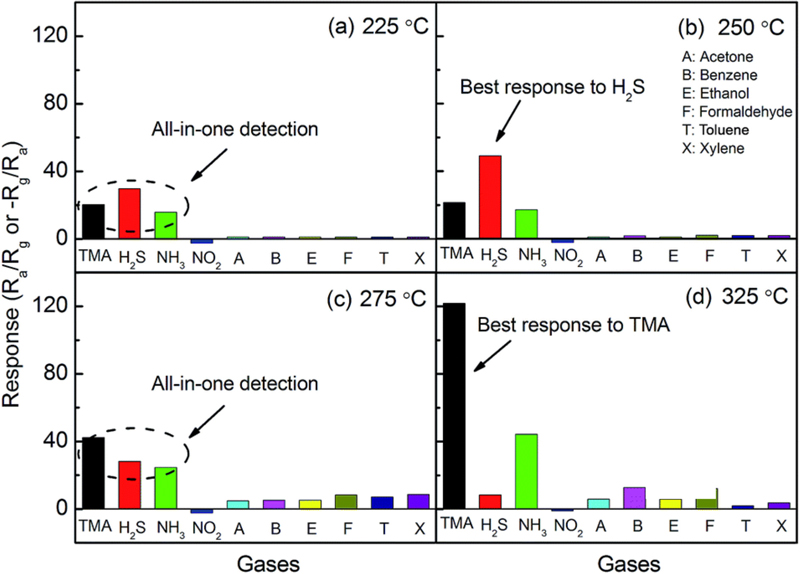
Selectivity of the MoO3-nanopaper sensor at different operation temperatures: 225°C, 250°C, 275°C, and 325°C. Reprinted with permission [27] Copyright 2017 Royal Society of Chemistry.
Ma et al. [29] explored a TMA sensor operating at room temperature using an MXene-MoO3 composite. Although MoO3 is widely used in metal-oxide-based gas sensors, the limitations of the operating temperature should be improved. Here, Ti3C2Tx, which has a high surface area and conductivity, was induced for the MXene-MoO3 hybrid structure. As shown in Figs. 6 (a-d), MoO3 nanofibers with Ti3C2Tx nanoflakes were successfully synthesized. The Ti3C2Tx-MoO3 composite exhibits superior performance in terms of sensitivity and response/recovery speed. Generally, the effects of p-n junctions, which facilitate enhanced conductivity and larger specific surface areas, arise from the unique structures of the 1D and 2D composites. Notably, the Ti3C2Tx-MoO3-based TMA sensor was investigated using a circuit diagram (Fig. 6 (f) and (g)).
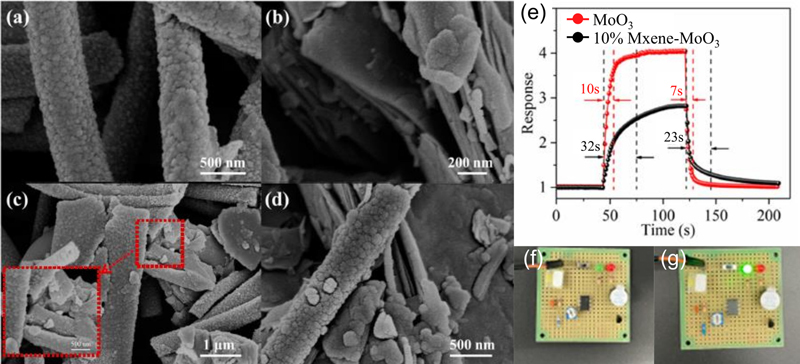
SEM images of (a) bare MoO3 nanofibers, (b) Ti3C2Tx nanoflakes, and (c, d) Ti3C2Tx-MoO3 hybrid structures. (e) Response and recovery performance of the Ti3C2Tx-MoO3 structure to 2 ppm TMA gas at 25°C. (f, g) Turning the alarm on and off in air and TMA conditions, respectively. Reprinted with permission [29] Copyright 2024, MDPI.
Hydrogen sulfide, a representative gas produced during the spoilage of most seafood and meat, is usually formed by the interaction of its enzymes with methionine (C5H11NO2S) or cysteine (C3H7NO2S). As spoilage accelerates, large amounts of H2S are released [30]. Chung et al. [31] reported improved sensitivity to hydrogen sulfide by depositing CrO3-based nanorods using glancing-angle deposition (GLAD). Shu et al. [32] reported a superior H2S gas sensor with adjusted organic-inorganic hybrid nanospheres (Figs. 7 (a) and (b)). For the effective sensing of H2S, Cu2+ ions help dissociate H2S into H+ and HS-(S2-). The dissociated molecules caused a change in resistance owing to their ease of reaction with polypyrrole and SnO2. Huo et al. [33] reported room temperature operating H2S sensors using MoS2 decorated In2O3 nanofibers. MoS2 decorated In2O3 nanofibers exhibited superior performance owing to their improved electron-transfer properties. Huo et al. validated the sensing performance of exhaled breath, suggesting the possibility of real-time monitoring using sensors in the future.
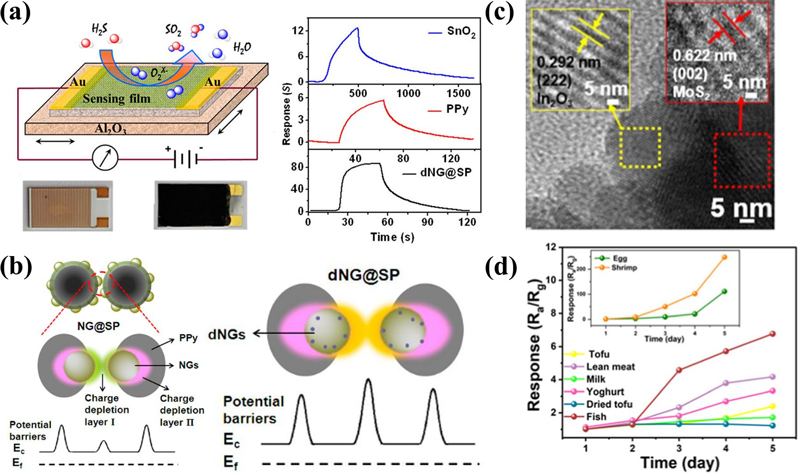
(a) Schematic of sensor with dynamic response curves for 50 ppm H2S. (b) Mechanism for effective H2S sensing using two different depletion regions. Reprinted with permission [32]. Copyright 2017. American Chemical Society. (c) HRTEM image of the In2O3@MoS2 heterostructure and (d) stability test of H2S concentration in foods. Reprinted with permission [33]. Copyright 2024. American Chemical Society.
3.2 ETC.
In addition to meat and fish, the freshness of fruits and vegetables and the maturity of wine are important factors for distribution and storage. Hence, developing highly sensitive sensors that can operate at room temperature is essential to commercialize these technologies.
Ethylene is mainly produced during the ripening of most fruits [34]. Fruit respiration interacts with atmospheric H2O, O2, and CO2, leading to the production of ethylene. Ethylene can accelerate ripening, potentially pushing the fruit from its optimal maturity to an undesirable stage of decay. Despite being one of the most important factors in agriculture, research on ethylene sensing remains limited because ethylene is nonpolar, making it difficult to detect. Jeong et al. [13] proposed an interference gas filtration method for an effective ethylene sensor. To improve the reactivity of ethylene, other interfering gases were preferentially oxidized to significantly reduce the response to interfering gases (Fig. 8 (a)). This approach resulted in a relative enhancement of the response towards ethylene, thereby demonstrating an improved selectivity for ethylene. Dang et al. [35] reported sensors with long-term stability based on hydrothermally synthesized Co3O4 nanorods. Although the sensitivity and selectivity were increased by the use of Pt catalysts, alternating noble metal catalysts and room-temperature operations have been studied for real-time monitoring. Jaisutti et al. [36] reported Ag-embedded ZnO nanoflowers for monitoring ripening (Fig. 8 (c)). Localized surface plasmon resonance and catalytic effects on the Ag nanoparticles improved their sensitivity, with the room temperature determined using UV light activation.
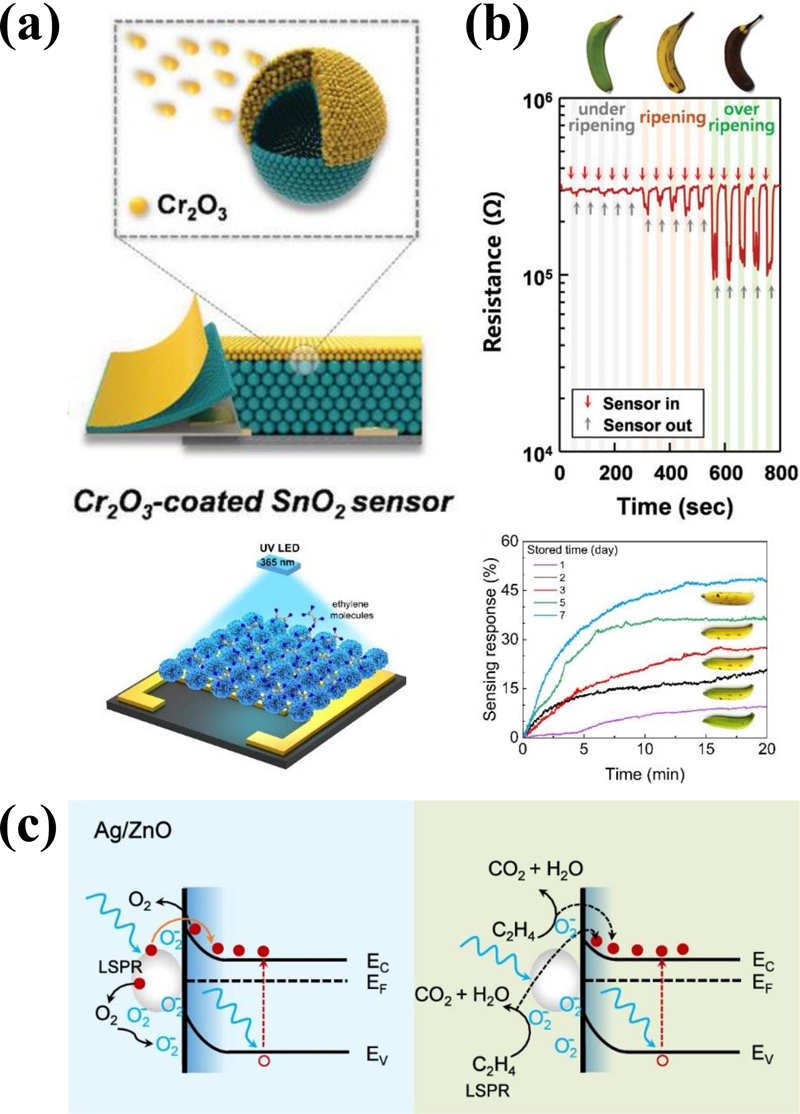
(a) Cr2O3-coated SnO2 sensors for ethylene sensing and (b) real-time monitoring of fruit freshness using a sensing module. Reprinted with permission [13]. Copyright 2020, WILEY. (c) Gas-sensing mechanism of Ag/ZnO sensors in air (blue) and ethylene (green) under UV illumination. Reprinted with permission [36]. Copyright 2024. American Chemical Society.
In addition to these gases, various volatile organic compounds (VOCs) are formed during spoilage and ripening [37]. Mahata et al. [38] demonstrated the potential for real-time monitoring of fruit freshness and spoilage by detecting the VOCs emitted from various fruits over time using SnO2 nanosheets. Additionally, Kovacs et al. [39] demonstrated the capability to detect various compounds, such as 2-octanol, decane, 1-propanol, and nbutanol—all produced during the fermentation of dairy products.
Various advanced studies on food monitoring have been conducted to improve the sensing performance. However, most gases emitted from spoiled foods are present at levels of only a few ppb. Moreover, several challenges, such as power consumption, the trade-off between sensitivity and operating temperature, independence from humidity, and low selectivity, must be addressed. To overcome these limitations, researchers have developed new technologies, such as sieving layers, that prevent water poisoning and enable sensors to operate at room temperature [40]. Platforms that integrate machine learning algorithms or memristor-based technologies are being explored to commercialize real-time monitoring systems [41]. In addition, MEMS gas-sensor arrays with enhanced sensing selectivity should be further investigated for monitoring systems considering the effects of various interfering gases [42].
4. CONCLUSION
In this paper, chemiresistive gas sensors are introduced and research trends in food monitoring are discussed. Amine-based compounds such as NH3, trimethylamine, and H2S play key roles in determining meat and fish spoilage. Metal-oxide-based chemiresistive gas sensors are usually combined with other active materials (i.e., heterostructures) to improve sensitivity and decorated with noble metal catalysts to enhance selectivity. However, the detection of low concentrations at room temperature remains a limitation.
Acknowledgments
This work was supported by a GIST Research Project grant funded by GIST.
REFERENCES
-
B. M. Oh, N. Y. Cho, E. H. Lee, S. Y. Park, H. J. Eun, and J. H. Kim, “Colorimetric and fluorometric bimodal amine chemosensor based on deprotonation-induced intramolecular charge transfer: Application to food spoilage detection”, J. Hazard. Mater., Vol. 465, p. 133150, 2024.
[https://doi.org/10.1016/j.jhazmat.2023.133150]

-
S. Y. Lee, S. J. Lee, D. S. Choi, and S. J. Hur, “Current topics in active and intelligent food packaging for preservation of fresh foods”, J. Sci. Food Agric., Vol. 95, No. 14, pp. 2799-2810, 2015.
[https://doi.org/10.1002/jsfa.7218]

-
S. Sonwal, K. S. Ranjith, S. Han, Y.-K. Han, M.-H. Oh, and Y. S. Huh, “Live-tracking of beef freshness by sub-ppb level ammonia detection using WS2/rGO nanoflakes incorporating edge site-enriched acidic sulfur”, J. Mater. Chem. A, Vol. 12, No. 18, pp. 11004-11019, 2024.
[https://doi.org/10.1039/D3TA07831K]

-
S. Lee, S. Kim, G. B. Nam, T. H. Eom, and H. W. Jang, “Chemoresistive Gas Sensors for Food Quality Monitoring”, J. Semicond. Technol. Sci., Vol. 22, Vol. 4, pp. 244-258, 2022.
[https://doi.org/10.5573/JSTS.2022.22.4.244]

-
N. M. Shaalan, F. Ahmed, O. Saber, and S. Kumar, “Gases in Food Production and Monitoring: Recent Advances in Target Chemiresistive Gas Sensors”, Chemosensors, Vol. 10, No. 8, p. 338, 2022.
[https://doi.org/10.3390/chemosensors10080338]

-
R. S. Andre, L. A. Mercante, M. H. M. Facure, R. C. Sanfelice, L. Fugikawa-Santos, T. M. Swager, and D. S. Correa, “Recent Progress in Amine Gas Sensors for Food Quality Monitoring: Novel Architectures for Sensing Materials and Systems”, ACS Sens., Vol. 7, No. 8, pp. 2104-2131, 2022.
[https://doi.org/10.1021/acssensors.2c00639]

-
S. Kim, G. H. Jeong, and S.-W. Kim, “Ethylene Gas Decomposition Using ZSM-5/WO3-Pt-Nanorod Composites for Fruit Freshness”, ACS Sustain. Chem. Eng., Vol. 7, No. 13, pp. 11250-11257, 2019.
[https://doi.org/10.1021/acssuschemeng.9b00584]

-
C. Almeida, J. O. Fernandes, and S. C. Cunha, “A novel paper for food spoilage detection”, Biosens. Bioelectron., Vol. 179, p. 113063, 2021.
[https://doi.org/10.1016/j.bios.2021.113063]

-
H. Kim, B. T. Trinh, K. H. Kim, J. Moon, H. Kang, K. Jo, R. Akter, J. Jeong, E.-K. Lim, J. Jung, H.-S. Choi, H. G. Park, O. S. Kwon, I. Yoon, and T. Kang, “Au@ZIF-8 SERS paper for food spoilage detection”, Biosens. Bioelectron., Vol. 179, p. 113063, 2021.
[https://doi.org/10.1016/j.bios.2021.113063]

-
A. T. John, K. Murugappan, D. R. Nisbet, and A. Tricoli, “An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring”, Sensors, Vol. 21, No. 7, p. 2271, 2021.
[https://doi.org/10.3390/s21072271]

-
L. Zeng, X. Xiao, H. Ye, D. Ma, and J. Zhou, “Fast visual monitoring of the freshness of beef using a smart fluorescent sensor”, Food Chem., Vol. 394, p. 133489, 2022.
[https://doi.org/10.1016/j.foodchem.2022.133489]

-
B. Kuswandi and A. Nurfawaidi, “On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness”, Food Control, Vol. 82, pp. 91-100, 2017.
[https://doi.org/10.1016/j.foodcont.2017.06.028]

-
S. J. Park, S. M. Lee, M.-H. Oh, Y. S. Huh, and H. W. Jang, “Food quality assessment using chemoresistive gas sensors: achievements and future perspectives”, Sustain. Food Technol., Vol. 2, No. 2, pp. 266-280, 2024.
[https://doi.org/10.1039/D3FB00196B]

-
S. Jeong, Y. K. Moon, T. Kim, S. Park, K. B. Kim, Y. C. Kang, and J. Lee, “A New Strategy for Detecting Plant Hormone Ethylene Using Oxide Semiconductor Chemiresistors: Exceptional Gas Selectivity and Response Tailored by Nanoscale Cr2O3 Catalytic Overlayer”, Adv. Sci., Vol. 7, No. 7, p. 1903093, 2020.
[https://doi.org/10.1002/advs.201903093]

-
G. Korotcenkov, “Metal oxides for solid-state gas sensors: What determines our choice?”, Mater. Sci. Eng. B, Vol. 139, No. 1, pp. 1-23, 2007.
[https://doi.org/10.1016/j.mseb.2007.01.044]

-
S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim, and T. W. Kim, “Recent advances in energy-saving chemiresistive gas sensors: A review”, Nano Energy, Vol. 79, p. 105369, 2021.
[https://doi.org/10.1016/j.nanoen.2020.105369]

-
S. H. Park, B.-Y. Kim, Y. K. Jo, Z. Dai, and J.-H. Lee, “Chemiresistive trimethylamine sensor using monolayer SnO2 inverse opals decorated with Cr2O3 nanoclusters”, Sens. Actuators B Chem., Vol. 309, p. 127805, 2020.
[https://doi.org/10.1016/j.snb.2020.127805]

-
M. Ma, X. Yang, X. Ying, C. Shi, Z. Jia, and B. Jia, “Applications of Gas Sensing in Food Quality Detection: A Review”, Foods, Vol. 12, No. 21, p. 3966, 2023.
[https://doi.org/10.3390/foods12213966]

-
S. Jiang and Y. Liu, “Gas sensors for volatile compounds analysis in muscle foods: A review”, TrAC Trends Anal. Chem., Vol. 126, p. 115877, 2020.
[https://doi.org/10.1016/j.trac.2020.115877]

-
H.-Y. Li, C.-S. Lee, D. H. Kim, and J.-H. Lee, “Flexible Room-Temperature NH3 Sensor for Ultrasensitive, Selective, and Humidity-Independent Gas Detection”, ACS Appl. Mater. Interfaces, Vol. 10, No. 33, pp. 27858-27867, 2018.
[https://doi.org/10.1021/acsami.8b09169]

-
R. Sankar Ganesh, E. Durgadevi, M. Navaneethan, V. L. Patil, S. Ponnusamy, C. Muthamizhchelvan, S. Kawasaki, P. S. Patil, and Y. Hayakawa, “Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres”, J. Alloys Compd., Vol. 721, pp. 182-190, 2017.
[https://doi.org/10.1016/j.jallcom.2017.05.315]

-
X. Li, W. Zeng, S. Zhuo, B. Qian, Q. Chen, Q. Luo, and R. Qian, “Highly Sensitive Room-Temperature Detection of Ammonia in the Breath of Kidney Disease Patients Using Fe2Mo3O8/MoO2@MoS2 Nanocomposite Gas Sensor”, Adv. Sci., Vol. 11, No. 32, p. 2405942, 2024.
[https://doi.org/10.1002/advs.202405942]

-
E. Yang, K. H. Park, T. Oh, and S. J. Kim, “Ultra-dense SnO2 QDs-decorated MXene nanosheets with high water dispersibility for rapid NH3 sensing at room temperature”, Sens. Actuators B Chem., Vol. 409, p. 135542, 2024.
[https://doi.org/10.1016/j.snb.2024.135542]

-
P. D. Hoat, V. K. Vo, S.-H. Bae, H.-J. Lim, D. T. H. Thao, P. T. Hung, N. M. Hung, N. V. Hoang, J.-H. Lee, and Y.-W. Heo, “Room-temperature sensing of NH3 gas using CsPb-Br3 thin films grown via dual-source evaporation”, J. Alloys Compd., Vol. 960, p. 170731, 2023.
[https://doi.org/10.1016/j.jallcom.2023.170731]

-
X. Tian, X. Cui, Y. Xiao, T. Chen, X. Xiao, and Y. Wang, “Pt/MoS2/Polyaniline Nanocomposite as a Highly Effective Room Temperature Flexible Gas Sensor for Ammonia Detection”, ACS Appl. Mater. Interfaces, Vol. 15, No. 7, pp. 9604-9617, 2023.
[https://doi.org/10.1021/acsami.2c20299]

-
N. Iqbal, A. Afzal, I. Khan, M. S. Khan, and A. Qurashi, “Molybdenum impregnated g-C3N4 nanotubes as potentially active photocatalyst for renewable energy applications”, Sci. Rep., Vol. 11, No. 1, p. 16886, 2021.
[https://doi.org/10.1038/s41598-021-96490-6]

-
H.-Y. Li, L. Huang, X.-X. Wang, C.-S. Lee, J.-W. Yoon, J. Zhou, X. Guo, and J.-H. Lee, “Molybdenum trioxide nanopaper as a dual gas sensor for detecting trimethylamine and hydrogen sulfide”, RSC Adv., Vol. 7, No. 7, pp. 3680-3685, 2017.
[https://doi.org/10.1039/C6RA26280E]

-
T.-H. Kim, J.-W. Yoon, Y. C. Kang, F. Abdel-Hady, A. A. Wazzan, and J.-H. Lee, “A strategy for ultrasensitive and selective detection of methylamine using p-type Cr2O3: Morphological design of sensing materials, control of charge carrier concentrations, and configurational tuning of Au catalysts”, Sens. Actuators B Chem., Vol. 240, pp. 1049-1057, 2017.
[https://doi.org/10.1016/j.snb.2016.09.098]

-
Z. Wang, W. Ma, J. Wei, K. Lan, S. Yan, R. Chen, and G. Qin, “Ultrasensitive Flexible Olfactory Receptor-Derived Peptide Sensor for Trimethylamine Detection by the Bending Connection Method”, ACS Sens., Vol. 7, No. 11, pp. 3513-3520, 2022.
[https://doi.org/10.1021/acssensors.2c01893]

-
J. Yan, Z. Zhao, X. Wang, and J. Xie, “Hydrogen sulfide in seafood: Formation, hazards, and control”, Trends Food Sci. Technol., Vol. 148, p. 104512, 2024.
[https://doi.org/10.1016/j.tifs.2024.104512]

-
J. H. Chung, Y.-H. Cho, J. Hwang, S. H. Lee, S. Lee, S.-H. Park, S. Sohn, D. Cho, K. Lee, and Y.-S. Shim, “Heterostructures of SnO2-Decorated Cr2O3 Nanorods for Highly Sensitive H2S Detection”, J. Sens. Sci. Technol., Vol. 33, No. 1, pp. 40-47, 2024.
[https://doi.org/10.46670/JSST.2024.33.1.40]

-
J. Shu, Z. Qiu, S. Lv, K. Zhang, and D. Tang, “Cu2+ -Doped SnO2 Nanograin/Polypyrrole Nanospheres with Synergic Enhanced Properties for Ultrasensitive Room-Temperature H2S Gas Sensing”, Anal. Chem., Vol. 89, No. 20, pp. 11135-11142, 2017.
[https://doi.org/10.1021/acs.analchem.7b03491]

-
Y. Huo, L. Qiu, T. Wang, H. Yu, W. Yang, X. Dong, and Y. Yang, “P–N Heterojunction formation: Metal Sulfide@Metal Oxide Chemiresistor for ppb H2S Detection from Exhaled Breath and Food Spoilage at Flexible Room Temperature”, ACS Sens., Vol. 9, No. 6, pp. 3433-3443, 2024.
[https://doi.org/10.1021/acssensors.4c00866]

-
A. Sholehah, K. Karmala, N. Huda, L. Utari, N. L. W. Septiani, and B. Yuliarto, “Structural effect of ZnO-Ag chemoresistive sensor on flexible substrate for ethylene gas detection”, Sens. Actuators A Phys., Vol. 331, p. 112934, 2021.
[https://doi.org/10.1016/j.sna.2021.112934]

-
T. T. L. Dang, V. D. N. Tran, T. X. Chu, M. H. Chu, V. D. Nguyen, and D. H. Nguyen, “Eco-friendly facile synthesis of Co3O4–Pt nanorods for ethylene detection towards fruit quality monitoring”, Sens. Actuators A Phys., Vol. 362, p. 114607, 2023.
[https://doi.org/10.1016/j.sna.2023.114607]

-
R. Jaisutti, S. Khemphet, S. Pudwat, N. Petchsang, Y.-H. Kim, T. Osotchan, and Z. Zhu, “UV-Induced Room-Temperature Ethylene Sensors Based on Ag-Decorated ZnO Nanoflowers for Fruit Ripeness Monitoring”, ACS Appl. Nano Mater., Vol. 7, No. 14, pp. 16575-16584, 2024.
[https://doi.org/10.1021/acsanm.4c02562]

-
S. D. Lawaniya, A. Awasthi, P. W. Menezes, and K. Awasthi, “Detection of Foodborne Pathogens Through Volatile Organic Compounds Sensing via Metal Oxide Gas Sensors”, Adv. Sens. Res., p. 2400101, 2024.
[https://doi.org/10.1002/adsr.202400101]

-
B. Mahata, S. Acharyya, S. Giri, T. Mahata, P. Banerji, and P. K. Guha, “Fruit Freshness Monitoring Employing Chemiresistive Volatile Organic Compound Sensor and Machine Learning”, ACS Appl. Nano Mater., Vol. 6, No. 24, pp. 22829-22836, 2023.
[https://doi.org/10.1021/acsanm.3c04138]

-
Z. Kovacs, Z. Bodor, J.-L. Zinia Zaukuu, T. Kaszab, G. Bazar, T. Tóth, and C. Mohácsi-Farkas, “Electronic Nose for Monitoring Odor Changes of Lactobacillus Species during Milk Fermentation and Rapid Selection of Probiotic Candidates”, Foods, Vol. 9, No. 11, p. 1539, 2020.
[https://doi.org/10.3390/foods9111539]

-
N. Ma, K. Suematsu, M. Yuasa, T. Kida, and K. Shimanoe, “Effect of Water Vapor on Pd-Loaded SnO2 Nanoparticles Gas Sensor”, ACS Appl. Mater. Interfaces, Vol 7, No. 10, pp. 5863-5869, 2015.
[https://doi.org/10.1021/am509082w]

-
B. Mahata, S. Acharyya, S. Giri, T. Mahata, P. Banerji, and P. K. Guha, “Fruit Freshness Monitoring Employing Chemiresistive Volatile Organic Compound Sensor and Machine Learning”, ACS Appl. Nano Mater., Vol. 6, No. 24, pp. 22829-22836, 2023.
[https://doi.org/10.1021/acsanm.3c04138]

-
T. Kim, T. H. Lee, S. Y. Park, T. H. Eom, I. Cho, Y. Kim, C. Kim. S. A. Lee, M.-J. Choi, J. M. Suh, I.-S. Hwang, D. Lee, I. Park, and H. W. Jang, “Drastic Gas Sensing Selectivity in 2-Dimensional MoS2 Nanoflakes by Noble Metal Decoration”, ACS Nano, Vol. 17, No. 5, pp. 4404-4413, 2023.
[https://doi.org/10.1021/acsnano.2c09733]