Fabrication of Au-In2O3 Thin/Thick-Film Gas Sensors and Their Sensing Characteristics for Toxic Gases
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This paper reports on highly sensitive and selective Au- In2O3 thin- and thick film gas sensors for detecting and measuring toxic gases. The used thin film was deposited on an alumina substrate using a high-frequency sputtering method, the sensor substrate with platinum heater and gold electrode was formed by screen printing, and the nano-catalyst material Au was deposited by thermal vapor deposition. The thick-film gas sensor was fabricated by screen printing using Au- In2O3 paste, and the sensor was finalized by annealing in air at 800oC. The target toxic gases used were acetaldehyde, dimethyl disulfide, and 2-methyl-1-propanol. The fabricated thin-film gas sensors showed excellent sensitivity to dimethyl disulfide, and the thick-film gas sensors showed a strong response to acetaldehyde. In particular, the thin-film sensors annealed at 800oC exhibited excellent sensing characteristics such as high sensitivity, fast recovery, stability, and linearity. Based on the surface oxidation reaction and electron transfer mechanism of the Au- In2O3 system, a sensing reaction mechanism and surface energy band model for 2-methyl-1-propanol were proposed.
Keywords:
Gas sensor, Toxic gas, Au-In2O3, Thin-thick film, Sputtering, Thermal evaporation1. INTRODUCTION
Detecting small amounts of toxic gases is a critical task in a variety of industries and in agriculture and indoor environmental regulatory response [1,2]. Metal oxide semiconductors such as SnO2, In2O3, TiO2, WO3, and ZnO are often used as base materials for the detection of oxidizing gases (Cl2, NO2, O3, CO2, etc.), reducing gases (CO, H2, SO2, CH4, etc.), and toxic gases (NH3, H2S, 2-methyl-1-propanol, dimethyl disulfide, acetaldehyde, etc.) [3,4]. Indium oxide as an n-type semiconductor with a band gap of 3.7 eV is a very promising material for toxic gas sensors due to its relatively low conduction activation energy [5,6]. The combination of metal oxides with a large surface area and noble metal catalysts enables a large number of reaction sites, which inspires its use as sensing materials in gas sensors.
In general, bulk Au has low activity as a heterogeneous catalyst; however, deposition of Au on metal oxides in the form of ultrasmall nanoparticles smaller than 5 nm can yield excellent activity and selectivity, and the catalytic properties are dependent on the metal oxide support [7]. The main factors affecting the catalytic activity of gold catalysts are the size and shape of the gold nanoparticles, the preparation method, the nature of the metal carrier (support), the gold-carrier interaction, and the oxidation state of the gold catalyst [8].
Steffes et al. [9] fabricated Au or Ti-In2O3 thin-film (Au: 2 nm, Ti: 40 nm, In2O3: 120 nm) gas sensors by radio-frequency (RF) sputtering on a SiO2/Si substrate and annealed them in O2 at 600°C. The 2 nm Au- In2O3 thin-film sensor exhibited good sensitivity to NO2 in gases mixed with CO, H2, SO2, and NH3 at operating temperatures of 350-400°C due to Au surface modification. Xing et al. [10] obtained Au-In2O3 powders through a coprecipitation method from In(NO3)3 and HAuCl4, and fabricated a Figaro-type gas sensor. A ceramic tube with a pair of gold electrodes printed on it was coated with a paste of sensing material and a small heating coil, such as a Ni-Cr alloy, was inserted into the ceramic tube to provide the operating temperature. The Au-In2O3 gas sensor, which had an increased 'spillover effect' due to the Au nanorods, was able to detect not only low acetone concentrations (10 ppb) at 250°C, but also low ethanol concentrations (50 ppb) at 400°C. Lee et al [11] prepared the sensor by forming In2O3 thin monolayers (20 − 200 nm) from In(NO3)x-H2O on glass and SiO2/Si substrates, annealing at 600°C, followed by electron beam evaporation of Au (1 − 10 nm) on the In2O3 layer to prepare the catalytic layer, and annealing the Au- In2O3 film at 500°C . The In2O3 thin film showed poor response to some gases, while the 3 nm Au- In2O3 thin film showed good response to volatile organic compounds (VOCs) (xylene, toluene, benzene) and ethanol. Bogdanov et al. [12] fabricated a thermal wire-type semiconductor gas sensor of a Pt spiral coil by applying H2PtCl6 and HAuCl4 in indium hydroxide solution and calcination at 800°C. Au- In2O3 showed good response to CO and C2H5OH, and the Pt catalyst was superior to Au catalyst for CO, C2H5OH, and NH3. Fu et al. [13] prepared rod-like Au-In2O3 powders using chemical synthesis from In(NO3)3 and HAuCl43H2O and fabricated gas sensors using the paste printing method. The sensor exhibited good sensitivity to CO gas and selectivity to CH4, H2, SO2, acetone, and propylene. Sun et al. [14] used the solvothermal method to obtain In2O3 nanocube cluster-embedded Au nanoparticles and fabricated a Figaro-type gas sensor, which was tested to detect CO, H2, CH4, NH3, NO2, H2S, and C2H6O at 200°C, with good responses for CO and C2H6O.
Pd-loaded indium oxide gas sensors fabricated by the hydrothermal method using indium nitride and Pd nanoparticles had a good response to NO2 and CH4 gases [15,16]. A Ag-loaded nanograin In2O3 synthesized by dissolving In(NO3)35H2O and AgNO3 was fabricated as a thick-film gas sensor. It provided a high-response formaldehyde (HCHO) gas sensor at room temperature [17]. Cu-doped In2O3 microspheres synthesized from InCl34H2O and Cu(NO3)23H2O by a solvothermal route were fabricated as thick-film gas sensors to detect NO2 gas [18]. In2O3–@Cu nanocomposite powders were synthesized with a sol–gel process with In(NO3)35H2O, Cu(NO3)23H2O, and graphene powders, and were fabricated as thermal wire semiconductor-type gas sensors. A sensor of a nanocomposite with 4-wt.% @Cu additive to In2O3 exhibited the highest sensitivity to NO2 [19].
Despite extensive research on Au-In2O3 sensors, to date, no comparative study of the sensing layers of sputtered thin films and printed thick films has been performed. In addition, no reports have addressed important process parameters such as sensing layer thickness, heat treatment, or catalyst addition methods. Furthermore, the mechanism underlying the surface oxidation reaction of toxic gases and the resulting electrical resistance change of Au-In2O3 semiconductors is still unclear. Experimental investigations are scarce, especially for toxic compound gases. Considering these gaps, in this study, we fabricated several sensors, analyzed their surface morphology, evaluated their toxic gas sensing properties, and investigated the energy band model and gas reaction mechanism on the Au-In2O3 surface.
In this study, we developed highly sensitive and selective Au- In2O3 thin film and thick film gas sensors using Au as a catalyst and In2O3 metal oxide semiconductor as the main sensing material for toxic gas detection. The In2O3 thin film was deposited on an alumina substrate using an RF sputtering method, and a platinum heater and Au or Pt electrodes were formed on one side of the Al2O3 substrate by screen printing, while the nano-catalyst Au in the thin film sensor was deposited using a thermal vapor deposition method. They were tested after annealing at 500, 600, 700, and 800°C. The In2O3 thick-film gas sensors were fabricated by screen printing using a Au-In2O3 paste. 2-methyl-1-propanol (C4H10O), dimethyl disulfide (C2H6S2), and acetaldehyde (C2H4O) were employed as toxic gases. The sensitivity, reaction time, adsorption and desorption, and linearity were tested. Some characteristics were compared between the thick-film and thin-film sensors. The morphologies of the Au-In2O3 thin- and thick-films were observed using an electron microscopy technique. Based on the gas sensing characteristic of Au-In2O3, the gas sensing reactions and mechanism could be explained using the surface-controlled/energy band model.
2. EXPERIMENTAL
2.1 Design of the Sensor and Process
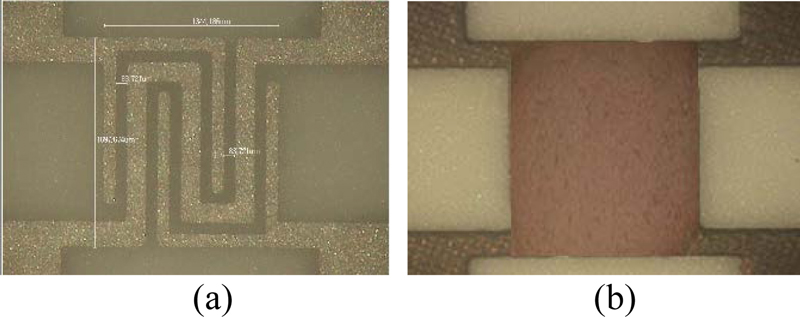
The sensor structure was designed as shown in Fig. 1, where (a) is a top view and (b) is a cross-sectional view. An alumina plate (4.0 3.5 0.25 mm3) was used as the sensor substrate. The electrodes, heater, and sensing layer were arranged in a flat shape. The heater (red), electrodes (blue), sensing layer (yellow), and catalyst layer (green) are stacked on the substrate, as shown in Fig. 1 (b). The electrode spacing is 85 μm. The heater and electrodes were fabricated using a screen printing technique with Pt paste on an alumina substrate. The thickness of both the heater and electrode was 150 μm. The resistance was 2.8 to 3.1 Ω. The In2O3 thin film sensing layer was deposited by RF sputtering, and the Au catalyst was prepared by thermal evaporation. The thick film sensing layer was prepared by screen printing Au-In2O3 paste.
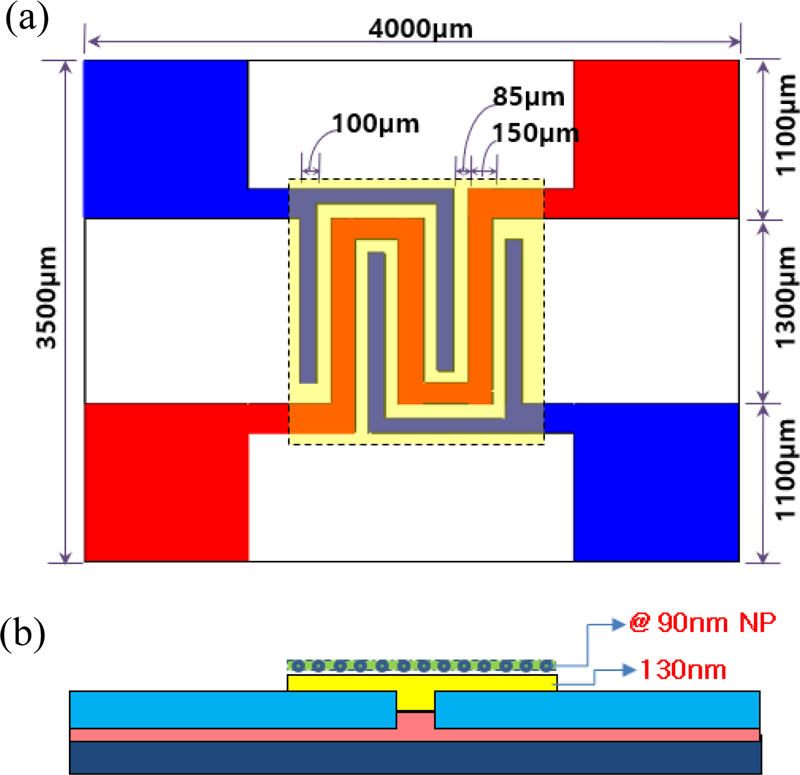
Configuration of unit thin-film sensor. (a) Top view, (b) cross section. Yellow: sensing material (In2O3), blue: electrode, red: Pt heater, green: catalyst.
Diluted standard toxic gases (RiGas Inc.) were used for the gas detection performance test. The output characteristics were measured by using a data output meter (Agilent/34907A) and configuring a voltage divider circuit.
2.2 Fabrication of the Thin-film Sensor
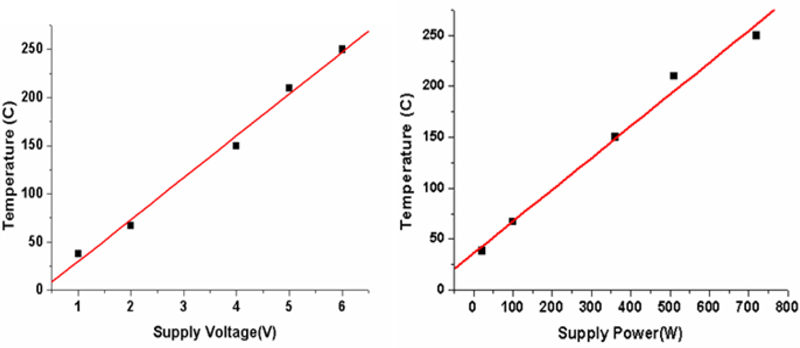
In2O3 thin films were fabricated by RF sputtering on alumina substrates using a 2-inch In2O3 target with 99.9% purity and an argon gas atmosphere (3.5 10−3 Torr), as shown in Fig. 1 (IBSSouth Bay Tech.). The sensing layer In2O3 had different film thicknesses depending on the deposition time: the thicknesses were 90 − 110 nm, 120 − 143 nm (≈131 nm), and 150 nm for deposition times of 20, 40, and 60 min, respectively. The thickness was verified by cross-sectional scanning electron microscopy (SEM). The catalytic material, Au, was deposited on the surface of the In2O3 sensing layer via thermal evaporation using a tungsten boat. Dozens of sensors were fabricated at a time on a single alumina substrate. The average electrical resistance of the thin film was about 1.1 ± 0.3 MΩ. Fig. 2 shows an optical picture of the fabricated prototype sensor. The lead wires were connected using a spot welder or conductive paste. The fabricated sensors were used to test the gas response characteristics. Fig. 3 is power-temperature relations of the sensor.
2.3 Thick-film Gas Sensor
For the thick-film gas sensor (Fig. 4), the sensing material, the thick film, was also fabricated from Au and In2O3. In2O3 powder was prepared by precipitation method using 99.9% indium chloride (Aldrich) and ammonia water as precipitant. HAuCl4-H2O (Aldrich) was used dissolved in hydrochloric acid and the proportion of the dissolved solution was varied to reveal the optimal catalyst quantitative ratio. The ratio of In2O3 to HAuCl4-H2O was best at 15:1. A paste of the sensing material was made by hand milling by mixing In2O3 powder, Au solution, and organic (PEG)/inorganic (Cu2O) binder. The sensor was then fabricated by screen printing the heater and electrode patterns on an alumina substrate, drying it in air, followed by a second drying at 150°C for 1 h and heat treatment at 800°C for 1 h. The thickness was measured to be 100 ± 12 μm and the resistance to be 5.2 ± 0.3 MΩ.
3. RESULTS AND DISCUSSION
3.1 Material Analysis
The SEM and mapping measurements of the 40-minute deposited thin film are shown in Fig. 5. By drawing a curve along the surface roughness of the alumina substrate, it can be seen that the thin film grows uniformly without any specific boundaries being formed (Fig. 5 (a)). As shown in Fig. 5 (b), the red color of the In2O3 thin film confirms that the film grew uniformly during the deposition time of 40 minutes. The Au deposited on the surface of the sensing layer via thermal evaporation shows a uniform distribution for the sample obtained using 14.5 mg of Au. As shown in Fig. 5 (a), circular Au particles (white dots) with an average size of about 90 nm are evenly deposited. Fig. 4 (b) shows that the In2O3 film (red) and Au (green) are also evenly distributed on this alumina.
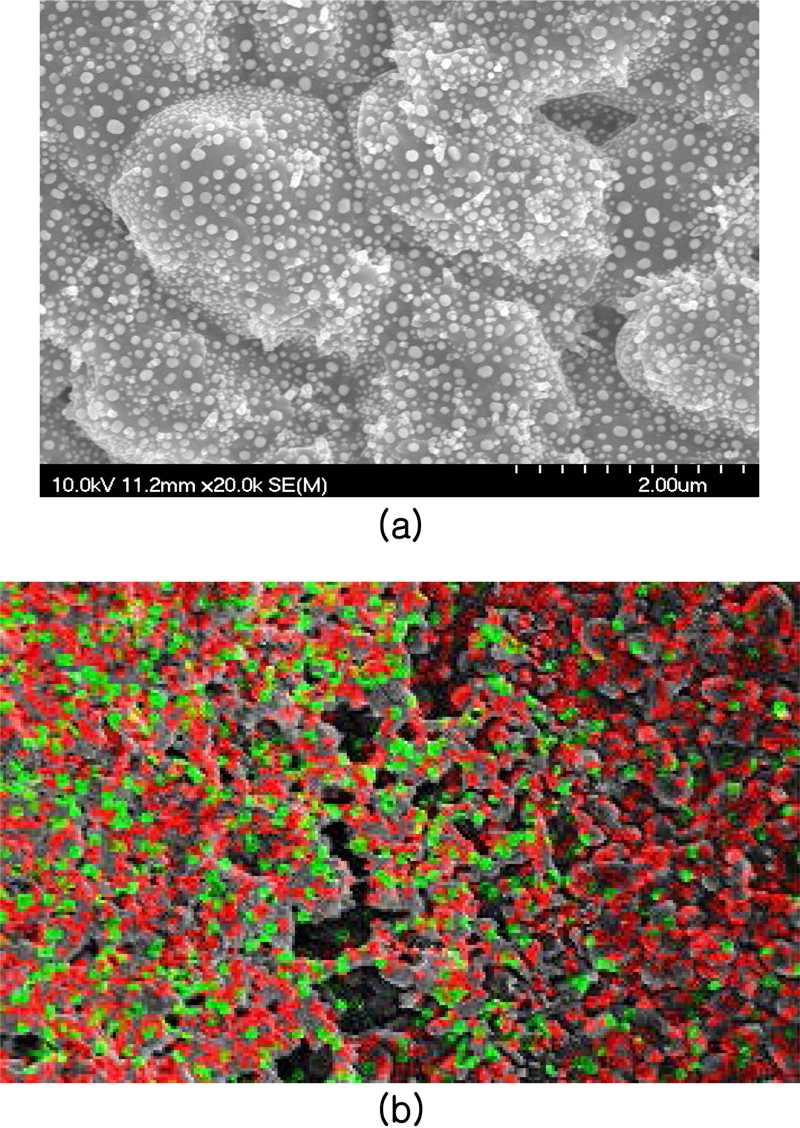
Au-In2O3 thin-film. (a) SEM image of Au-In2O3 and Au particles (white dots), (b) map, green : Au, red : In2O3, gray : Al2O3.
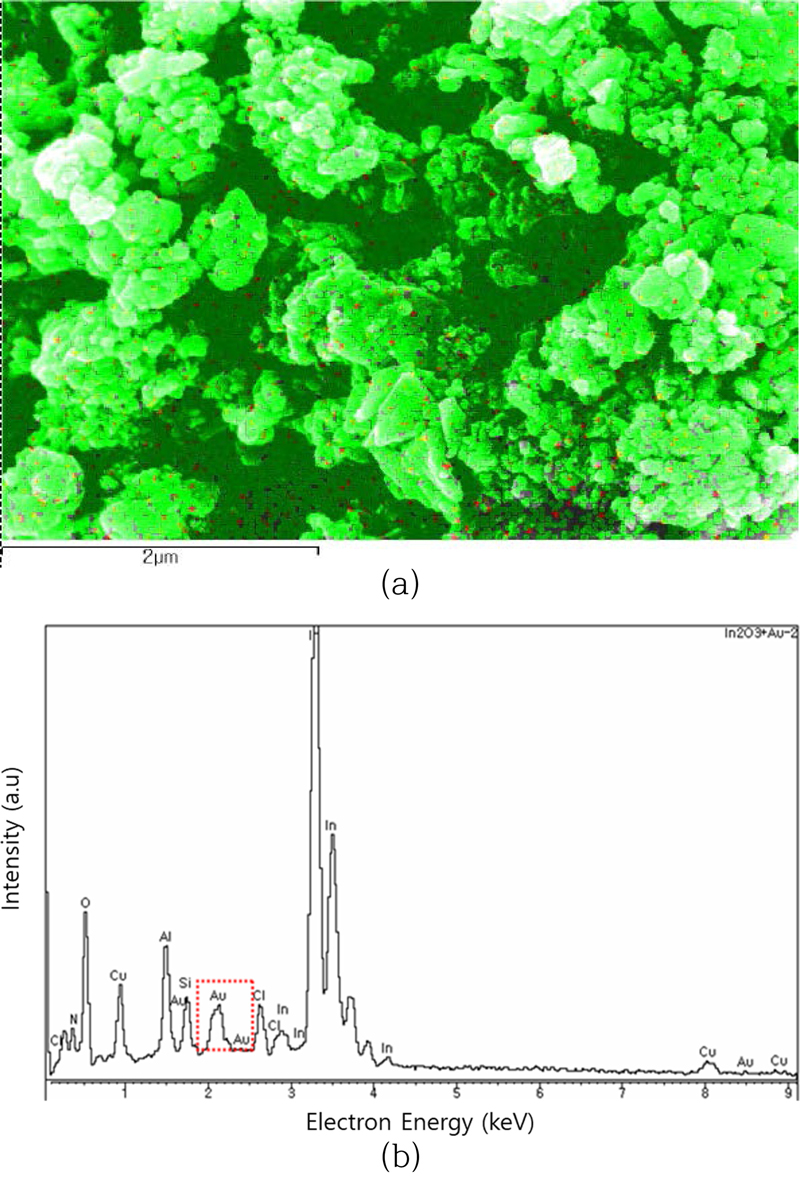
Fig. 6 shows SEM energy dispersive X-ray (EDX) spectroscopy of a sensing material of Au- In2O3 powder used in a thick film sensor. Fig. 6 (a) shows the In2O3 powder morphology and the added Au catalyst (~20 nm/red dot). Most of the powder is agglomerated to form large particles. Fig. 6 (b) shows the EDX peak composition analysis of the powder. Indium, the sensing material, appears as the main peak, followed by Au, the catalyst. Some impurities from the substrate, Al and Si, are also visible. Peaks of Cu added as an inorganic binder and Cl residual from gold chloride are also observed. Structures such as Cu2O are expected to affect the gas sensing reaction.
3.2 Sensing Characteristics
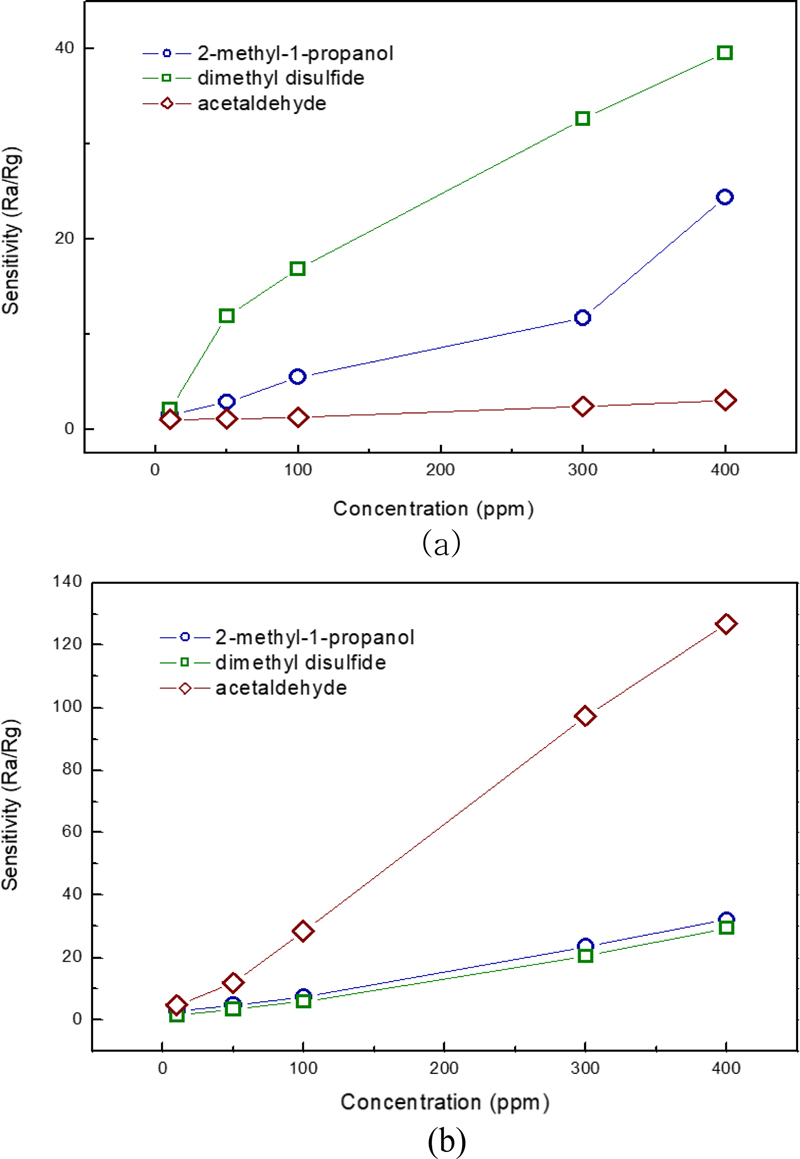
Fig. 7 shows the sensitivity of the fabricated Au-In2O3 gas sensor to different toxic gas concentrations. The operating temperature of the sensor is 250°C. The test gases are acetaldehyde, dimethyl disulfide, and 2-methyl-1-propanol. The measurement range is from 10 to 400 ppm. Fig. 7 (a) shows the measurement results of the thin-film sensor, which showed a low response for acetaldehyde, moderate sensitivity for dimethyl disulfide, and high sensitivity for 2-methyl-1-propanol. Fig. 6 (b) shows the sensitivity measurements of the thick-film sensor. Acetaldehyde showed a very strong sensing response. The other two gases showed a moderate response.
The sensitivity is measured at T90 (output height value at 90% of the output peak time). The reason why the acetaldehyde sensitivity of the thick film sensor is superior to that of the thin film is attributed to the difference in thickness of the In2O3 sensing layer [about 770 times (100 μm/131 nm)], i.e., the difference in specific surface area (reaction area) and the amount of oxygen adsorbed. In addition, the difference in particle size of the Au catalyst (20 nm/90 nm) and the difference in surface distribution of the thin film containing Au particles and the bulk fraction of the thick film are also expected to affect the sensitivity. Cu2O and SiO2 added as binders in the thick film are also believed to affect the adsorption and desorption of gases, enhancing the sensitivity to a specific gas, acetaldehyde.
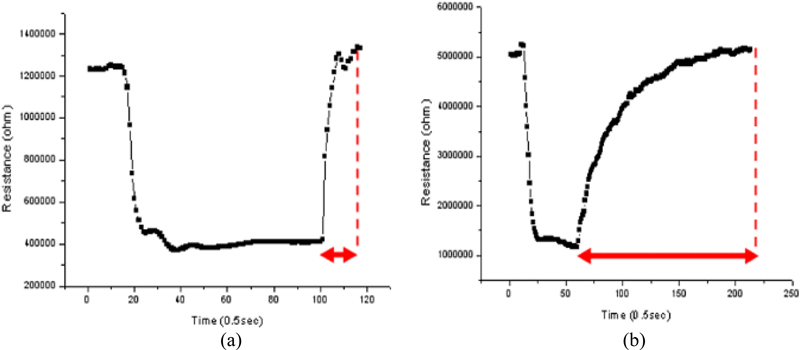
As shown in Fig. 8, the response of the toxic gas sensor (acetaldehyde gas, 10 ppm, 250°C) shows that there is a large difference in the recovery rate of the signal to return to the initial level after gas detection. The adsorption reaction time between the toxic gas and ionized adsorbed oxygen is similar for both thin and thick films at 5 − 7 seconds, but there is a large difference in the desorption reaction. The thick film sensor has a period of about 160 seconds in this example, and the response curve gradually returns to the initial signal level, whereas the thin film sensor shows a fast recovery characteristic with a short period of about 10 seconds.
This difference in recovery rate can be attributed to the thickness of the sensing layer. A thick film has more pores and necks than a thin film. In this case, even if the reactant gas is exhausted and fresh oxygen is introduced, the longer lag time for oxygen to penetrate to the bottom of the thick film results in slow re-adsorption of oxygen ions. This slow re-adsorption causes the sensor resistance to slowly reach its initial level.
Fast recovery is one of the important characteristics in the practical use of gas sensors, especially since high-performance sensors may be applied in environments where repeated detection is required, and fast recovery can be critical for accurate detection analysis. The reproducibility of sensor characteristics also seems to favor thin-film sensors over thick-film sensors.
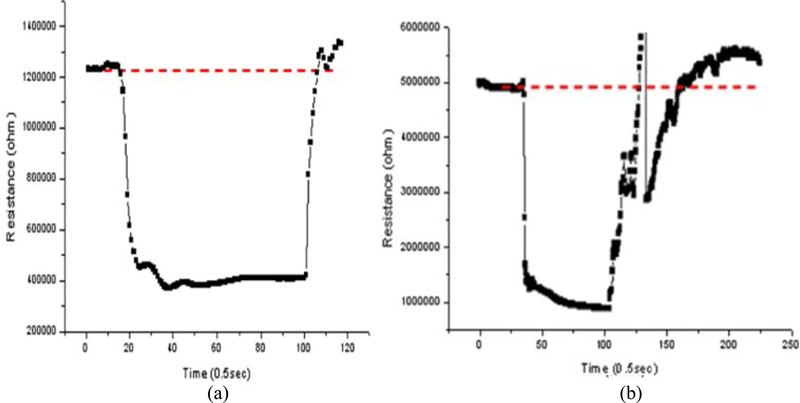
In general, laboratory-level sensor studies have shown that the characteristics of the sensors can vary depending on the process conditions. The response curve characteristics of the thin-film sensors tended to have an overall stable baseline compared to the thick-film sensors. As shown in Fig. 9 (a), the curve returning to the initial signal level (baseline) after reaction with the sensing gas (acetaldehyde) is stable for the thin-film sensors, with the signal level approaching the presented level.
Thick film sensors typically exhibit a stable adsorption response, but often show unstable fluctuations in the signal after gas detection, as shown in Fig. 9 (b). The slow and unstable overflow desorption reaction after the adsorption reaction can be attributed to some secondary reactions of the toxic gas inside the thick film. These characteristics can lead to errors in the sensing characteristics and negatively affect the product yield. Therefore, thick film sensors exhibit higher sensing reactivity (acetaldehyde) than thin film sensors, but are inferior to thin film sensors in terms of signal curve stability.
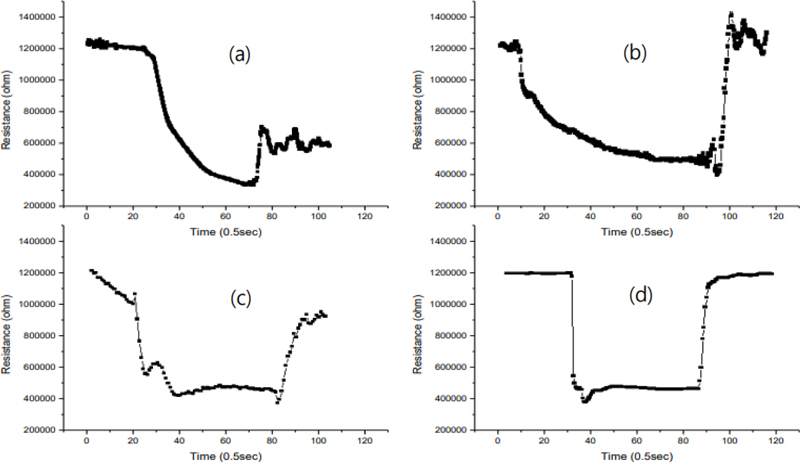
The sensing characteristic curve of a thin film sensor as a function of the heat treatment temperature of the sensing material is shown in Fig. 10. After the deposition of the sensing material and catalyst, heat treatment is an important process step to stabilize the properties of the sensing material. The heat treatment temperatures were 500, 600, 700, and 800°C. The sensor that underwent 800°C annealing exhibited good sensitivity, best stability, and ideal adsorption and desorption response for 2-methyl-1-propanol (10 ppm, 250°C), as shown in Fig. 10 (d). When the annealing temperature is lower than 800°C, the sensing tends to slow down or the adsorption/desorption reaction characteristics become unstable, as shown in Fig. 10. This suggests that a sufficiently annealed Au-In2O3 film is stable and optimized for crystallization, film texture (pores and columns), and inter-granular surface (size and neck). The samples can be divided into low-temperature annealed (500 − 700°C) and high-temperature annealed (800°C) samples. In the former case, the deposited grains that make up the thin film form grain boundary contacts, while in the latter case, grain necks are formed. Grain boundary contacts have a high potential barrier and high resistance, while grain necks have almost no potential barrier and very low electrical resistance [20-22]. These factors may be responsible for the good sensitivity and stable adsorption/desorption of sensors that have undergone high-temperature heat treatment.
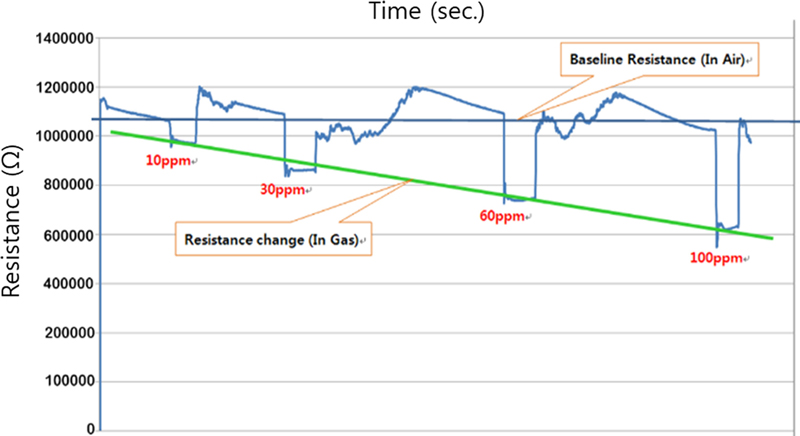
As shown in Fig. 11, a series of tests with different concentrations of 2-methyl-1-propanol showed that the sensor responds well even at concentrations as low as 10 ppm. In addition, the measurement results across concentrations show linearity, demonstrating the potential for use as a thin-film gas sensor. Reliability and reproducibility are also excellent. The response time and recovery time are very good, but the baseline tends to wobble slightly, which can be attributed to moisture and temperature effects from outside air entering the sensor.
Generally, when metal oxide semiconductors such as SnO2, In2O3, and TiO2 are exposed to air, lattice metals of the surface interact with adsorbed oxygen atoms [23], which could be described by
| (1) |
| (2) |
| (3) |
| (4) |
Pure In2O3-based sensors were investigated by X-ray photoelectron spectroscopy (XPS) to confirm the ratio of the chemisorbed oxygen [24]. The O2−, O−, and O2− ratios of In2O3 were determined to be 82.13, 1.83, and 16.05%, respectively. Changes within 5% were observed even when a catalyst (5% Ag) was added.
The adsorbed oxygen atoms trap free electrons from the conduction band on the In2O3 surface. Following the above reaction, the consumption of free electrons from the In2O3 surface region creates a depletion layer (space charge layer, Debye length) that lacks carriers. This leads to an increase in the electrical resistance of the In2O3 semiconductor. The width of the depletion layer, which depends on the material, catalyst, grain size, grain boundaries, reaction gas, and sensor operating temperature, is critical to the sensing characteristics.
The reactions of 2-methyl-1-propanol (C4H10O), dimethyl disulfide (C2H6S2), and acetaldehyde (C2H4O) gases at the In2O3 surface can be expressed as
| (5) |
| (6) |
| (7) |
When the Au- In2O3 material is in contact with gas, reactive oxygen ions directly react with gas molecules according to the above Eq. (5) − (7). During the decomposition and oxidation of carbon-containing molecules, common intermediates such as meth-, ethoxide-, acetate-, OH-groups, and adsorbed oxygen can be formed on the surface of metal oxides such as SnO2, In2O3, and ZnO [25]. However, in this thin film sensor study, these intermediates are not expected to be generated because a sufficient amount of Au catalyst was used and the operating temperature of the sensor was as high as 250°C.
Following the above reactions, the trapped free electrons are released back into the In2O3 bulk, narrowing the depletion layer, lowering the potential barrier, and reducing the electrical resistance of the Au-In2O3 film. The sudden change in resistance becomes a sensing signal for the gas sensor, indicating the presence and concentration of gas.
To improve gas sensing properties, catalysts with spillover effects are commonly used to diffuse electrons between surfaces. The electron effect due to the different work functions of Au (5.1 eV) and In2O3 (4.8 eV) contributes to the formation of Schottky junctions at the interface of these two materials, which allows electrons to transfer from gold to In2O3 via gas adsorption/desorption [25-27]. Thus, Au catalysts contribute to increased sensitivity, reduced operating temperature, and improved selectivity.
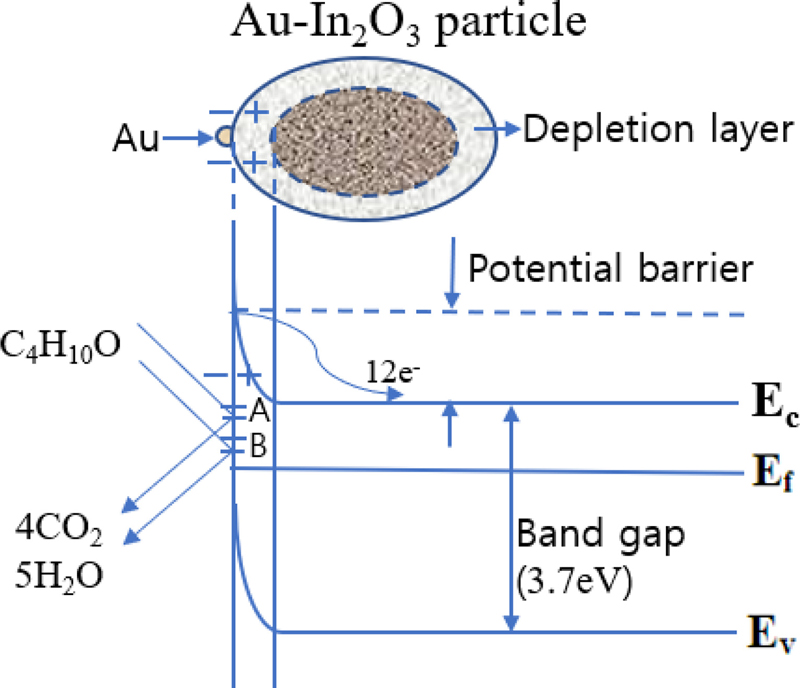
Fig. 12 schematically shows the electronic energy band diagram of the Au-In2O3 sensor surface exposed to a reducing gas (2-methyl-1-propanol/C4H10O) based on the above description and Eq. (1) − (5). Au-In2O3 sensors work by trapping or releasing free electrons (12e−) in the conduction band when exposed to air or reducing gas. The depletion layer (space charge layer) and potential barrier vary, and the electronic conductivity changes depending on the adsorbed oxygen-gas reaction process.
4. CONCLUSIONS
In this work, we developed highly sensitive and selective Au- In2O3 thin film and thick film gas sensors for toxic gases with Au as the catalyst and In2O3 metal oxide semiconductor as the main sensing material. The In2O3 thin film was deposited on an alumina substrate using an RF sputtering method, and a platinum heater and gold electrode were formed on one side of the Al2O3 substrate by screen printing, and the nano-catalyst material Au was deposited using a thermal vapor deposition method. The In2O3 thick-film gas sensor was fabricated by screen printing using Au-In2O3 paste. 2-methyl-1-propanol (C4H10O), dimethyl disulfide (C2H6S2), and acetaldehyde (C2H4O) were employed as toxic gases.
The thin-film gas sensor showed good sensitivity to dimethyl disulfide and 2-methyl-1-propanol. The thick-film gas sensor showed a very strong response to acetaldehyde. Moderate responses were observed for the other two gases. The superior sensitivity of the thick film sensor compared to the thin film sensor can be attributed to the difference in thickness of the In2O3 sensing layer (total amount of pores/specific surface area). The addition of Au catalyst (particle size/internal distribution) and Cu2O/SiO2 as binder may also have affected the adsorption and desorption of gases. The thin film gas sensor annealed at 800°C showed satisfactory results in terms of sensitivity, response time, adsorption/desorption, and linearity.
REFERENCES
-
A. I. Ayesh, “Metal/metal-oxide Nanoclusters for Gas Sensor Applications”, J. Nanomater., Vol. 16, pp. 1-17, 2016.
[https://doi.org/10.1155/2016/2359019]

-
A. Soussi, E. Zero, R. Sacile, D. Trinchero, and M. Fossa, “Smart Sensors and Smart Data for Precision Agriculture: A Review”, Sensors, Vol. 2, No. 8, pp. 2647-2679, 2024.
[https://doi.org/10.3390/s24082647]

-
Y. Masuda, “Recent advances in SnO2 nanostructure based gas sensors”, Sens. Actuators B Chem., Vol. 364, pp. 1-27, 2022.
[https://doi.org/10.1016/j.snb.2022.131876]

-
Z. Wang, L. Zhu, J. Wang, R. Zhuang, P. Mu, J. Wnag, and W. Yan, “Advanced in functional guest materials for resistive gas sensors”, RSC Adv., Vol. 12, pp. 24614-24632, 2022.
[https://doi.org/10.1039/D2RA04063H]

-
A. Walsh, J. Da Silva, S.-H. Wei, C. Korber, A. Klein, L. F. J. Piper, A. Demasi, K. Smith, G. Panaccione, P. Torelli, D. J. Payne, A. Bourlange, and R. G. Egdell, “Nature of the Band Gap of In2O3 Revealed by First-Principles Calculations and X-Ray Spectroscopy”, Phys. Rev. Lett., Vol. 100, No. 16, pp. 167402(1)-167402(4), 2008.
[https://doi.org/10.1103/PhysRevLett.100.167402]

-
B. Horoz, S. Tuna Yıldırım, B. Soltabayev, A. Ateş, S. Acar, and M. A. Yıldırım, “Effect of SILAR cycle on gas sensing properties of In2O3 thin flms for CO gas sensor”, J. Mater. Sci. Mater. Electron., Vol. 35, No. 2, pp. 163(1)-163(14), 2024.
[https://doi.org/10.1007/s10854-024-11970-5]

-
M. Haruta, “Size and support-dependency in the catalysis of gold”, Catal. Today, Vol. 36, No. 1, pp. 153-166, 1977.
[https://doi.org/10.1016/S0920-5861(96)00208-8]

-
A. S. Alshammari, “Heterogeneous Gold Catalysis: From Discovery to Applications”, Catalysts, Vol. 9, No. 5, pp. 402(1)-402(22), 2019.
[https://doi.org/10.3390/catal9050402]

-
H. Steffes, C. Imawam, F. Solzbacher, and E. Obermeier, “Enhancement of NO2 sensing properties of In2O3-based thin films using an Au or Ti surface modification”, Sens. Actuators B Chem., Vol. 78, No. 1-3, pp. 106-112, 2001.
[https://doi.org/10.1016/S0925-4005(01)00799-7]

-
R. Xing, L. Xu, J. Song, C. Zhou, Q. Li, D. Liu, and H. Wei Song, “Preparation and Gas Sensing Properties of In2O3/Au Nanorods for Detection of Volatile Organic Compounds in Exhaled Breath”, Sci. Rep., Vol. 5, p. 10717, 2015.
[https://doi.org/10.1038/srep10717]

-
C. S. Lee, Z. Dai, H. Y. Li, Y. M. Jo, B. Y. Kim, H. G. Byun, I. Hwang, and J. H. Lee, “Highly discriminative And sensitive detection of volatile organic compounds For monitoring indoor air quality using pure and Au-Loaded 2D In2O3 inverse opal thin films”, Sens. Actuators B Chem., Vol. 273, pp. 1-18, 2018.
[https://doi.org/10.1016/j.snb.2018.06.011]

- P. A. Bogdanov, V. V. romanovskaya, and M. I. Ivanovskaya, “Semiconductor sensors for Ammonia determination”, Russ. J. Appl. Chem., Vol. 72, No. 3, pp. 462-465, 1999.
-
H. Fu, C. Hou, F. Gu, D. Han, and Z. Wang, “Facile preparation of rod-like Au/In2O3 nanocomposites exhibiting high response to CO at room temperature”, Sens. Actuators B Chem., Vol. 243, pp. 516-524, 2017.
[https://doi.org/10.1016/j.snb.2016.11.162]

-
Y. Sun, Z. Zhao, R. Zhou, P. Li, W. Zhang, K. Suematsu, and J. Hu, “Synthesis of In2O3 nanocubes, nanocube clusters, and nanocubesembedded Au nanoparticles for conductometric CO sensors”, Sens. Actuators B Chem., Vol. 345, p. 130433, 2021.
[https://doi.org/10.1016/j.snb.2021.130433]

-
J. Hu, Y. Liang, Y. Sun, Z. Zhao, M. Zhang, P. Li, W. Zhang, Y. Chen, and S. Zhuiykov, “Highly sensitive NO2 detection on ppb level by devices based on Pd-loaded In2O3 hierarchical microstructures”, Sens. Actuators B Chem., Vol. 252, pp. 116-126, 2017.
[https://doi.org/10.1016/j.snb.2017.05.113]

-
Y. Zhao, S. Wang, W. Yuan, S. Fan, Z. Hua, Y. Wu, and X. Tian, “Selective detection of methane by Pd-In2O3 sensors with a catalyst filter film”, Sens. Actuators B Chem., Vol. 328, p. 129030, 2021.
[https://doi.org/10.1016/j.snb.2020.129030]

-
S. Zhou, M. Chen, Q. Lu, Y. Zhang, J. Zhang, B. Li, H. Wei, J. Hu, H. Wang, and Q. Liu, “Ag Nanoparticles Sensitized In2O3 Nanograin for the Ultrasensitive HCHO Detection at Room Temperature”, Nanoscale Res. Lett., Vol. 14, pp. 365(1)-365(11), 2019.
[https://doi.org/10.1186/s11671-019-3213-6]

-
X. Hu, L. Tian, H. Sun, B. Wang, Y. Gao, P. Sun, F. Liu, and G. Lu, “Highly enhanced NO2 sensing performances of Cu-doped In2O3 hierarchical flowers”, Sens. Actuators B Chem., Vol. 221, pp. 297-304, 2015.
[https://doi.org/10.1016/j.snb.2015.06.080]

-
A. Khort, Y. Haiduk, I. Taratyn, D. Moskovskikh, K. Podbolotov, A. Usenka, N. Lapchuk, and V. Pankov, “High-performance selective NO2 gas sensor based on -In2O3–graphene–Cu nanocomposites”, Sci. Rep., Vol. 13, p. 7834, 2023.
[https://doi.org/10.1038/s41598-023-34697-5]

-
N. Yamazoe and N. Miura, “Some basic aspects of semiconductor gas sensors”, Chem. Sens. Technol., Vol. 4, pp. 19-42, 1992.
[https://doi.org/10.1016/B978-0-444-98680-1.50007-3]

-
N. M. Ahmed, F. A. Sabah, H. I. Abdulgafour, A. Alsadig, A. Sulieman, and M. Alkhoaryef, “The effect of post annealing temperature on grain size of indium-tinoxide foroptical and electrical properties improvement”, Results Phys., Vol. 13, pp. 102159-102165, 2019.
[https://doi.org/10.1016/j.rinp.2019.102159]

-
Y. Yang, B. Maeng, D. G. Jung, J. Lee, Y. Kim, J. B. Kwon, H. K. An, and D. Jung, “Annealing effects on SnO2 thin film for H2 gas sensing”, Nanomaterials, Vol. 12, No. 18, pp. 3227-3239, 2022.
[https://doi.org/10.3390/nano12183227]

-
M. Iwamoto, “Characterization oxygen adsorbates on semiconductive oxides”, Chem. Sens. Technol., Vol. 4, pp. 63-83, 1992.
[https://doi.org/10.1016/B978-0-444-98680-1.50009-7]

-
G. Heiland and D. Kohl, “Physical and chemical aspects of oxidic semiconductor gas sensor”, Chem. Sens. Technol., Vol. 1, pp. 15-38, 1988.
[https://doi.org/10.1016/B978-0-444-98901-7.50007-5]

-
P. V. Tong, L. H. Minh, N. V. Duy, and C. M. Hung, “Porous In2O3 nanorods fabricated by hydrothermal method for an effective CO gas sensor”, Mater. Res. Bulletin, Vol. 137, p. 111179, 2021.
[https://doi.org/10.1016/j.materresbull.2020.111179]

-
Z. Li, Y. Huang, S. Zhang, W. Chen, Z. Kuang, D. Ao, W. Liu, and Y. Fu, “A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit”, J. Hazard. Mater., Vol. 300, pp. 167-174, 2015.
[https://doi.org/10.1016/j.jhazmat.2015.07.003]

-
K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont, and S. Phanichphant, “Semiconducting metal oxides as sensors for environmentally hazardous gases”, Sens. Actuators B Chem., Vol. 160, pp. 580-591, 2011.
[https://doi.org/10.1016/j.snb.2011.08.032]