Fabrication of Nanopillars-Based Raman Substrates for Maximizing SERS Effects: Focusing on Advancement and Applications
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Plasmonic nanopillars are highly promising substrates for surface-enhanced Raman scattering (SERS) because of their simple fabrication process, cost-effectiveness, and efficient nanogap formation. These characteristics render them ideal for biosensing and environmental monitoring. This review highlights the advancements in SERS substrates utilizing nanopillars fabricated via electrochemical methods with a focus on strategies to enhance the sensitivity, stability, and reproducibility. Key approaches include the electrochemical fabrication of silver nanopillars and introduction of their growth mechanisms. To address the oxidation issues of Ag nanopillars, bimetallic nanopillars developed through galvanic replacement are discussed, along with hybrid nanostructures designed to increase the nanogap density and electric field amplification. These structures have demonstrated excellent detection capabilities for biomarkers, such as amyloid beta and uric acid, as well as environmental toxins, such as cadmium, mercury, and thiram. In conclusion, electrochemical nanopillars offer a versatile, scalable, and cost-effective platform for high-performance SERS sensors, strongly supporting their potential practical applications in biosensing and environmental monitoring.
Keywords:
SERS, Nanopillar, Biosensor, Environmental sensor1. INTRODUCTION
Raman spectroscopy is a powerful, nondestructive analytical technique commonly referred to as molecular fingerprinting, owing to its ability to generate unique spectral patterns for individual molecules [1-3]. When a monochromatic light source is projected onto a sample, the vibrational modes associated with molecular bonds undergo characteristic shifts, enabling precise molecular identification. This high specificity has facilitated the application of Raman spectroscopy in diverse analytical fields, including forensic science and artwork authentication [4,5]. Despite its advantages, Raman spectroscopy suffers from an inherently low scattering probability of approximately 1 in 108, resulting in a weak signal intensity and limited sensitivity. These constraints hinder its utility as a high-sensitivity sensing tool [6,7]. Surfaceenhanced Raman scattering (SERS) has been developed to overcome these limitations. By significantly enhancing the scattering probability and sensitivity, SERS has expanded the application of Raman spectroscopy to various domains such as biosensing and the detection of toxic substances.
SERS is primarily attributed to two mechanisms: the electromagnetic (EM) and chemical (CM) enhancement mechanisms [8,9]. EM arises from localized surface plasmon resonance (LSPR) phenomena that occur within the nanogaps of metallic nanostructures under incident electromagnetic fields, leading to a significant amplification of the local electric field [8,10]. In contrast, CM is attributed to charge transfer processes and chemical interactions between the analyte and substrate surface [9,11]. In terms of Raman signal amplification, EM typically results in enhancements exceeding the order of 106, while CM contributes more modestly, with enhancements on the order of 102. Consequently, EM generally exhibits a significantly higher amplification effect than that of CM. These two mechanisms synergistically contribute to the enhanced Raman signal observed in SERS, with the EM playing a dominant role in most cases and CM providing additional sensitivity through specific molecular interactions [11].
The design of SERS substrates is of paramount importance for maximizing the SERS effect. A SERS substrate, typically comprising metallic nanostructures, must exhibit the LSPR effect, possess uniform nanogaps, and facilitate interactions with target molecules [12,13]. From a material perspective, the low imaginary refractive index of nanomaterials minimizes electric field losses in the surrounding medium. Consequently, materials with low imaginary refractive indices, such as noble metals (Au, Ag, Al, Cu), bimetals (core-shell, alloys), and more recently, two-dimensional transition metal dichalcogenides, such as MoS2, have been extensively explored for SERS substrate development [14-16]. Geometrically, structures such as nanoparticles, nanopillars, and nanopores, which are conducive to nanogap formation, have been widely investigated as SERS substrates [17-19]. Among these, nanopillar structures have garnered significant attention because of their unique structural advantages for enhancing the SERS effect. Nanopillars feature high aspect ratios and surface areas, providing abundant sites for target molecule adsorption that contribute to superior reproducibility and uniformity compared with other structures [20-23]. Moreover, the nanogaps between adjacent nanopillars enhance the electromagnetic field, enabling the detection of molecules at low concentrations, and thus facilitating high-sensitivity detection [18]. However, conventional nanopillar structures are typically fabricated using microelectromechanical system (MEMS) processes, which result in high production costs, complex fabrication procedures [24], and extended processing times, posing challenges for large-scale implementation.
In contrast, EC-based nanopillars provide a cost-effective and straightforward alternative for overcoming these limitations. Their unique ability to facilitate nanogap formation has resulted in superior performance compared to traditional MEMS-based SERS structures [18].
EC-based nanopillars are typically formed via the reduction of Ag ions to form nanostructures. However, Ag is highly susceptible to oxidative stress, which reduces its long-term stability. To address this issue, a bimetallic nanopillar structure was developed by coating Ag with Au, a metal with high oxidation resistance. This not only enhanced the structural stability, but also improved the SERS performance through the bimetallic effect. Despite these advancements, the random growth of EC-based nanopillars introduces variability in nanogap formation, leading to reproducibility issues. This limitation was addressed by integrating nanoparticles with nanopillars to form hybrid nanostructures, which significantly improved the reproducibility and overall performance. This review aims to explore the growth mechanisms, applications, and limitations of EC-based nanopillars, while highlighting innovative strategies developed to overcome these challenges (Fig. 1).
Schematic illustration of the stepwise advanced electrochemical (EC) fabricated nanopillar SERS substrate.
investigated as SERS substrates [17-19]. Among these, the nanopillar structure has garnered significant attention due to its unique structural advantages in enhancing the SERS effect.
Nanopillars feature high aspect ratios and surface areas, providing abundant sites for target molecule adsorption, which contributes to superior reproducibility and uniformity compared to other structures [20-23]. Moreover, the nanogaps between adjacent nanopillars enhance the electromagnetic field, enabling detection of molecules at low concentrations and thus facilitating high-sensitivity detection [18]. However, conventional nanopillar structures are typically fabricated using micro-electromechanical systems (MEMS) processes, which result in high production costs and extended processing times, posing challenges for large-scale implementation.
Conventional MEMS processes are associated with high production costs and complex fabrication procedures [24]. In contrast, EC-based nanopillars provide a cost-effective and straightforward alternative, overcoming these limitations. Their unique ability to facilitate nanogap formation has demonstrated superior performance compared to traditional MEMS-based SERS structures [18].
EC-based nanopillars are typically formed by reducing Ag ions to create nanostructures. However, Ag is highly susceptible to oxidative stress, which poses a challenge for long-term stability. To address this issue, a bimetallic nanopillar structure was developed by coating Ag with gold, a metal with high oxidation resistance. This not only enhanced the structural stability but also improved the SERS performance through the bimetallic effect. Despite these advancements, the random growth nature of EC-based nanopillars introduces variability in nanogap formation, leading to reproducibility issues. This limitation was addressed by integrating nanoparticles with nanopillars to form hybrid nanostructures, which significantly improved reproducibility and overall performance. This review aims to explore the growth mechanisms, application cases, and limitations of EC-based nanopillars, while highlighting innovative strategies developed to overcome these challenges (Fig. 1).
2. EC FABRICATED NANOPILLAR
2.1 Growth mechanism of EC fabricated nanopillars
The EC-fabricated nanopillars were synthesized via EC reactions. In detail, the EC comprises a working electrode (WE), counter electrode (CE), and reference electrode (RE) [25,26]. Each electrode plays a distinct role in the EC process: the WE facilitates the reduction reaction of the Ag plating solution on its surface, CE completes the circuit by converting the open circuit into a closed circuit, and RE provides a stable reference for the potential applied to the WE. To ensure consistency in the reaction volume and surface area, a Polydimethylsiloxane (PDMS) mold was attached to the WE and a constant potential was applied to induce the reduction process [27].
The voltage applied to the WE was higher than the reduction potential of the Ag ions, which introduced an overpotential. Without an overpotential, the reaction proceeds under equilibrium conditions, resulting in the growth of a thin film instead of the desired pillar structure [18].
As shown in Figs. 2 (a)–(c), applying an overpotential allowed Ag seeds to form on the substrate surface until a charge of approximately 50 mC was reached. Beyond 100 mC, these seeds serve as nucleation sites for the anisotropic growth of pillar structures [18,28]. However, when the charge exceeds 150 mC, excessive Ag deposition occurred, leading to overgrowth and interconnections between the pillars. This reduced the number of nanoscale gaps and diminished the SERS effect, as illustrated in Fig. 2 (d–e).
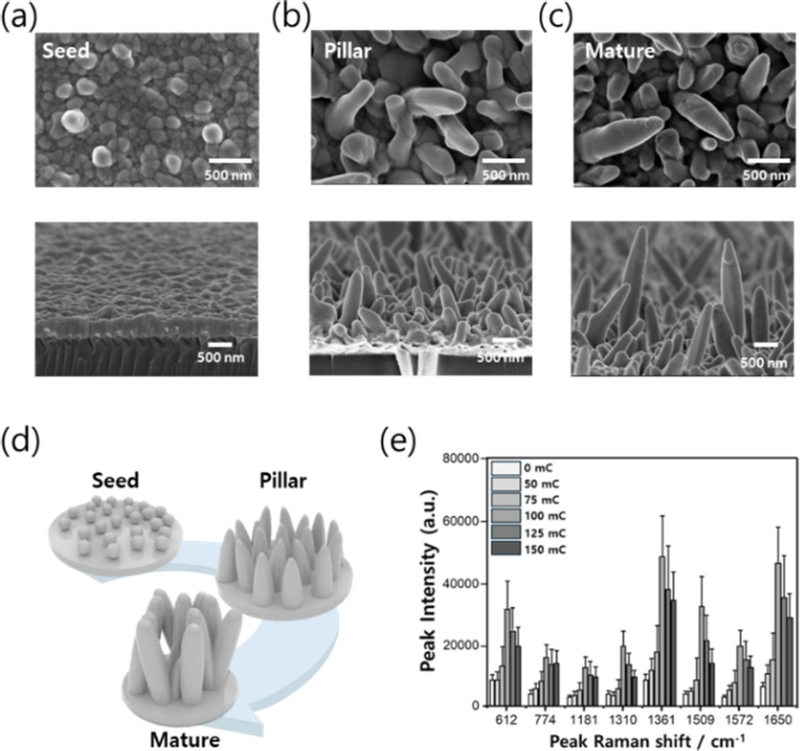
SERS performance of Ag nanopillars at different growth stages. (a–c) Scanning electron microscopy (SEM) images of Ag nanopillars corresponding to their growth levels. (d) Illustrated images according to the growth stage of each SERS substrate. (e) SERS intensity according to surface charge density (growth length).
2.2. Application of EC fabricated nanopillar
Optimized Ag nanopillars have been used in SERS-based applications for the detection of disease biomarkers and toxic substances [27,28,31]. Among these, the detection of vitamin D3, a biomarker associated with immune function, infectious diseases, and diabetes, is representative. Recently, vitamin D3 has also been identified as being closely associated with COVID-19 [29], highlighting the importance of monitoring and managing its levels in the body. Liquid chromatography is a traditional method for detecting vitamin D3. Chromatography and immunoassays were performed as reported previously [30]. However, these approaches often require complex sample preparation and centrifugation steps, presenting practical challenges. To overcome these limitations, a simple and highly sensitive SERS-based method was employed [28].
In this study, the concentration of vitamin D3 was measured by analyzing the SERS signal of methylene blue (MB), which is indirectly linked to vitamin D3. A sandwich assay approach was used, in which an aptamer selectively bound to vitamin D3 was immobilized on Ag nanopillars. Because vitamin D3 itself does not generate a strong SERS signal, indirect detection was achieved by conjugating MB to vitamin D3 via 4-Phenyl-1,2,4-triazoline-3,5-dione (PTAD), a molecule capable of binding to MB (Fig. 3 (a)). The SERS signal of MB attached to the vitamin D3–aptamer complex immobilized on the Ag nanopillars was analyzed to determine the vitamin D3 concentration in the body.
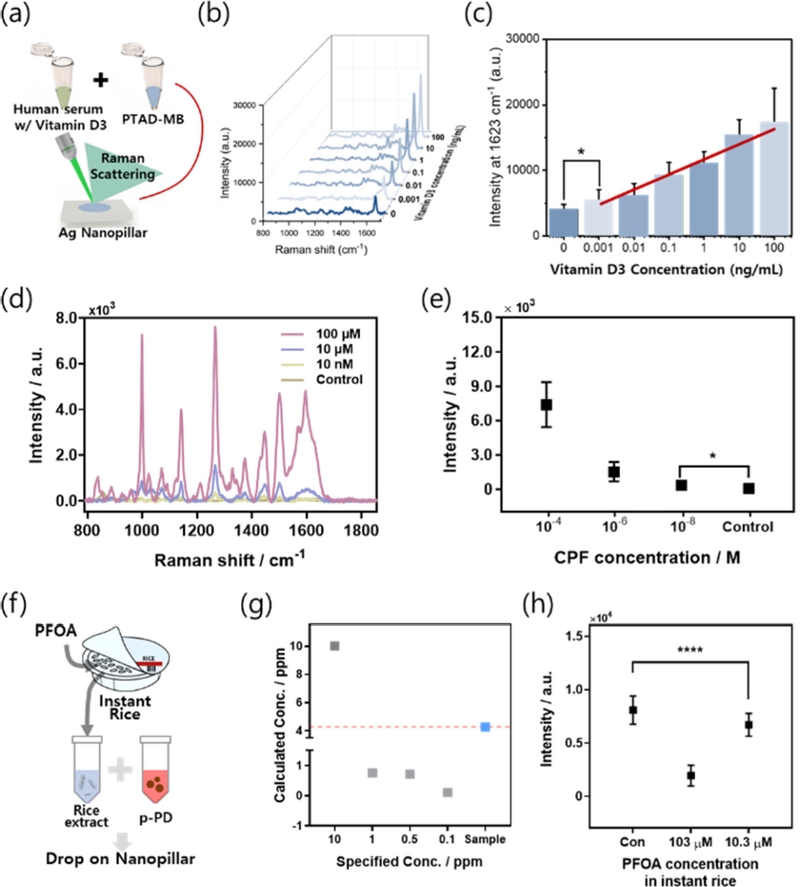
Application of Ag nanopillar SERS sensor. (a) A schematic of the Ag nanopillar SERS sensor designed for detecting vitamin D3 in human serum. (b–c) SERS spectra and corresponding intensities as a function of vitamin D3 concentration in human serum. (d–e) SERS spectra and intensities based on CPF concentration. (f) A schematic illustration of the Ag nanopillar SERS sensor for detecting PFOA in rice. (g) Liquid chromatography–mass spectrometry (LC–MS) analysis comparing a real reagent (blue) and a standard PFOA reagent (gray) based on their quantities. (h) SERS intensities of the Ag nanopillar sensor as a function of PFOA concentration.
Using Ag nanopillars, vitamin D3 was detected in 10% serum with a linear response in the range 1–100 ng/mL [28]. This detection range encompasses both deficient (<20 ng/mL) and excess (>100 ng/mL) levels of vitamin D3 in the body, demonstrating the capability of this sensor for wide-range detection [28]. Furthermore, this study serves as a foundational step toward the highly sensitive detection of other disease biomarkers.
The use of Ag nanopillars to detect toxic substances has been reported, with a focus on sensors for chlorpyrifos (CPF) and perfluoroalkyl substances (PFAS). CPF, an organophosphorus insecticide, causes neurological and immune disorders with symptoms, such as headaches, vomiting, visual disturbances, and paralysis. PFAS, historically used in nonstick cookware coatings, are now banned because of their toxicity. Among these, perfluorooctanoic acid (PFOA) is a non-biodegradable and known carcinogen that was recently classified as a Group 1 carcinogen. This underscores the critical need for highly sensitive detection methods for CPF and PFOA across diverse environments.
Conventional methods for detecting CPF, such as molecularly imprinted polymer-based EC techniques, often have low sensitivities and limited performance. To overcome these limitations, a highly sensitive and selective SERS detection approach was employed [32]. CPF was sampled onto the Ag nanopillars using a drop-casting method, followed by SERS analysis. As shown in Fig. 3 (d–e), CPF concentrations ranging from 10 nM to 100 μM in aqueous solutions were successfully detected. Notably, this detection range was extended to concentrations lower than the toxic threshold of CPF [33].
Conventional methods such as EC and MS have been widely used for the detection of PFOA [34]. However, these methods often suffer from low sensitivity or require complex sample preparation, making it challenging to detect PFOA in real-world environments [35]. To address these limitations, a SERS sensor was developed by self-assembling p-phenylenediamine (pPD) into spherical nanoparticles. When exposed to PFOA, the pPD nanoparticles disintegrate because of the peptide bonds and surfactant properties of PFOA. Using this mechanism, PFOA was successfully detected at concentrations as low as 1.28 pM under laboratory conditions, with a dynamic detection range of 10 pM to 1 mM in aqueous solution [31]. Given the historical use of PFOA in nonstick cookware coatings, it is highly likely that PFOA residues will be found in fried rice or similar dishes.
Therefore, PFOA was adsorbed onto instant rice at varying concentrations and SERS measurements were performed (Fig. 3 (f)). The SERS signals were compared to those obtained through conventional LC–MS, demonstrating the ability to detect PFOA concentrations down to 10.3 μM (Fig. 3 (g–h)).
These results highlight the potential of Ag nanopillars and SERS-based sensors for high-sensitivity detection of toxic substances, providing an effective alternative to conventional analytical techniques.
3. BIMETALLIC NANOPILLAR
3.1 Coating mechanism of galvanic replacement based bimetallic nanopillar
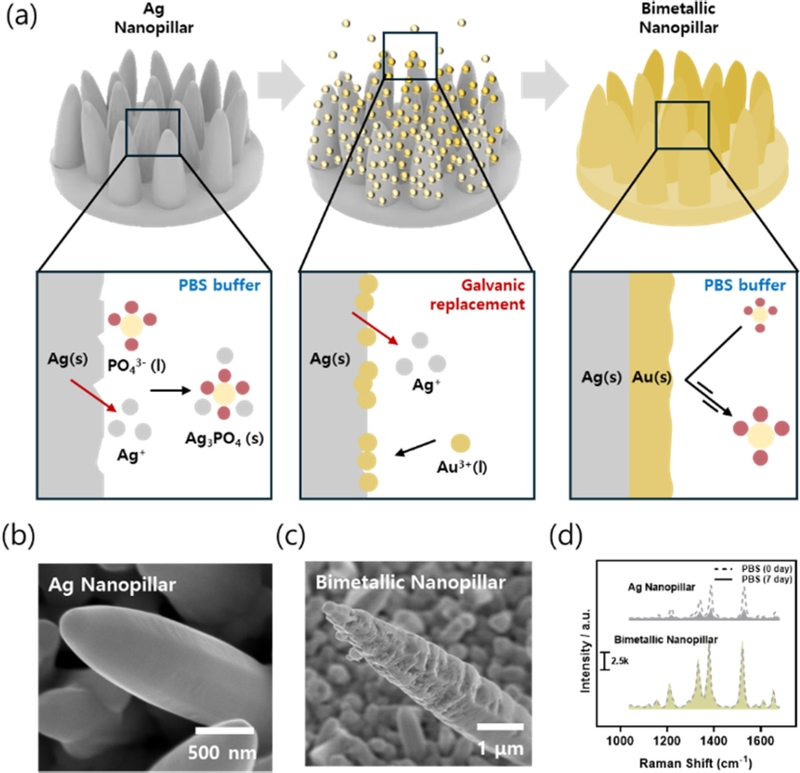
One of the primary limitations of EC fabricated Ag nanopillars is their susceptibility to surface oxidation. This oxidation compromises the long-term stability of the sensor, especially in biological samples containing high concentrations of NaCl and metal ions, which can corrode the Ag surface [36]. To address this issue, it is necessary to fabricate Au nanopillars due to the superior stability of gold. One of the primary limitations of the EC-fabricated Ag nanopillars is their susceptibility to surface oxidation. This oxidation compromises the long-term stability of the sensor, particularly in biological samples containing high concentrations of NaCl and metal ions that can corrode the Ag surface [36]. To address this issue, it is necessary to fabricate Au nanopillars because of their superior stability. However, Au requires a higher reduction potential and longer reaction time than Ag, making its growth more challenging [37]. Consequently, studies utilizing bimetallic nanopillars in which Au is coated onto Ag nanopillars have been reported [36,38].
Galvanic replacement is a solution-based electroless plating method that enables the coating of Au onto Ag surfaces without requiring additional EC equipment. This process leverages the standard reduction potential difference between Au and Ag, whereby Ag is oxidized, and Au³+ is reduced, leading to a replacement reaction [36]. Notably, for every single Au³+ ion reduced, three Ag atoms are oxidized, resulting in the formation of nanoscale gaps on the surface during the reaction [38].
| (1) |
When galvanic replacement occurred on the Ag nanopillars, nanogaps were created on the surface (Fig. 4 (b–c)). These nanogaps contributed to enhanced stability, even in oxidative stress environments, owing to the presence of Au (Fig. 4 (a)). Under conditions mimicking a biological environment, such as phosphate-buffered saline (PBS), Ag nanopillars exhibited a rapid decline in SERS performance over seven days. In contrast, the bimetallic nanopillar structure demonstrated sustained performance over the same period (Fig. 4 (d)).
3.2 Applications of bimetallic nanopillars in SERS sensor
Bimetallic nanopillars have been successfully applied in the detection of heavy metal ions in food and biomolecules, with reported studies targeting plasma-based mercury (Hg²+), amyloid beta oligomers (Aβ1-42), and cadmium ions (Cd²+) [36,38].
In particular, Hg²+ is a highly toxic metal associated with Minamata disease, neurological disorders, and kidney damage [39]. This is particularly concerning owing to its bioaccumulation in the food chain, which necessitates its detection in food products [40]. The detection strategy leverages the specific chelation reaction between Hg²+ and thymine (T) bases in DNA, forming T-Hg-T mismatches [41]. DNA was immobilized on the bimetallic nanopillar surface to enable Hg²+ detection. The method was validated using Hg²+ solutions prepared from canned tuna and tuna sashimi extract, where distinct SERS intensity maps allowed visual discrimination of concentrations (Fig. 5). The sensor exhibited a detection range of 10 nM to 100 μM, demonstrating its high sensitivity and effectiveness in food safety applications (Fig. 5).
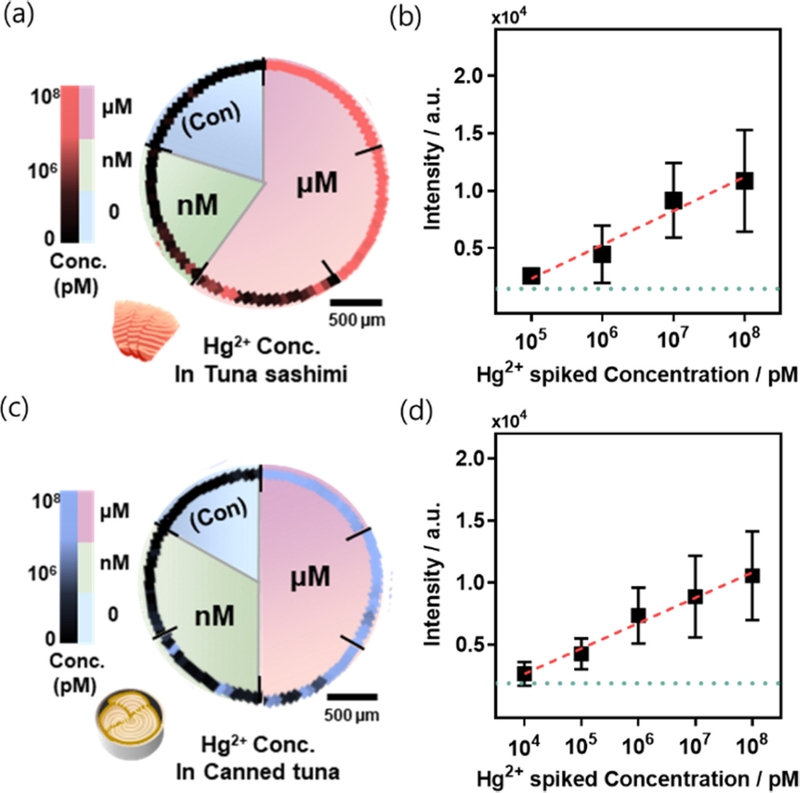
Application of bimetallic nanopillar SERS sensor. (a) SERS map image of Hg2+ at various concentrations in tuna sashimi. (b) SERS intensities of Hg2+ at different concentrations in tuna sashimi. (c) SERS map image of Hg2+ at various concentrations in canned tuna. (d) SERS intensities of Hg2+ at different concentrations in canned tuna.
4. HYBRID NANOSTRUCTURE
4.1 SERS enhancement mechanism of hybrid nanostructure
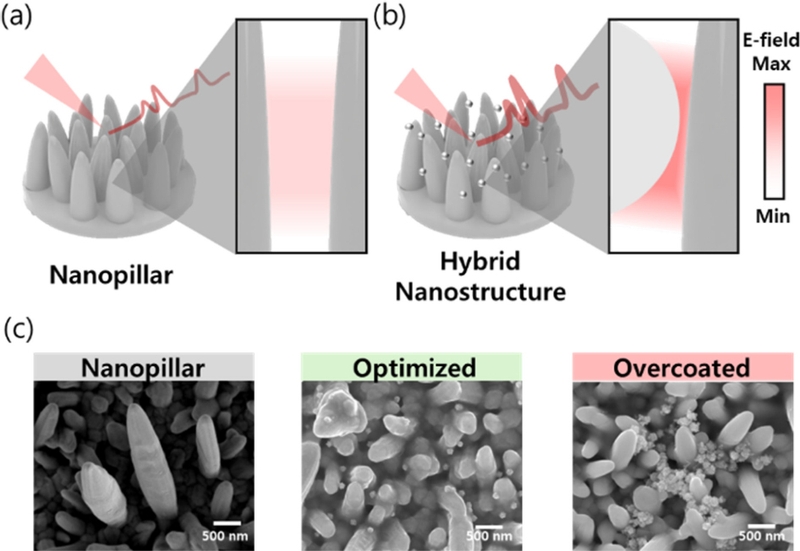
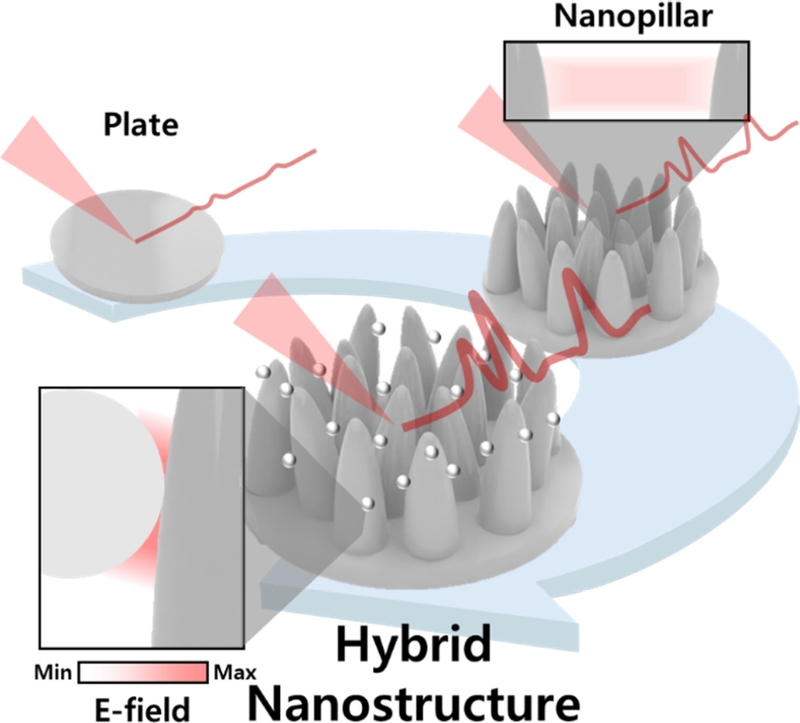
Conventional nanopillar structures face limitations in uniformly creating nanogaps between all pillars, which affects their sensitivity and reproducibility. To address these challenges, hybrid nanostructures combining nanopillars with nanoparticles have been developed, which offer improved sensitivity and uniformity to SERS substrates [42]. Hybrid nanostructures increase the number of nanogaps through strategic coupling of nanoparticles with nanopillar structures, enabling the fabrication of highly reproducible and uniform nanostructures (Fig. 6 (a–b)). The optimal number of nanoparticles on the nanopillar surface was determined through SEM imaging and electromagnetic simulation (Fig. 6 (c)) [42]. Simulations revealed that conventional nanopillars amplify the electric field only at the interpillar gaps, whereas properly arranged nanoparticles amplify the field across multiple regions. However, excessive nanoparticle deposition (overcoating) reduces the number of nanogaps, leading to decreased field enhancement. These findings emphasize the importance of precise nanoparticle arrangement on the nanopillar surfaces to maximize SERS signal amplification.
4.2 Applications of hybrid nanostructures in SERS sensor
Hybrid nanostructures have been used to detect thiram and uric acid, demonstrating their potential in biosensing and environmental monitoring [42,43].
Thiram is a pesticide that causes respiratory and skin diseases upon ingestion [44]. To detect thiram, an SERS sensor combining Ag nanopillars with bumpy Au nanoparticles was designed. The bumpy Au nanoparticles provide an increased surface area and additional nanogaps, resulting in enhanced SERS performance [44]. Thiram has been reported in milk because of pesticide-contaminated crops consumed by cows [44]. Using this hybrid nanostructure, thiram was detected in milk at concentrations ranging from 1 pM to 100 μM (Fig. 7 (a–c)). The sensor demonstrated the capability to detect thiram levels below harmful thresholds, thus confirming its suitability for real-world applications in food safety and environmental monitoring.
Uric acid, a purine metabolic byproduct, serves as a biomarker of kidney function and gout monitoring [45]. A SERS sensor combining bimetallic nanopillars with Au nanoparticles was developed to detect uric acid in biofluids containing both metabolites and minerals.
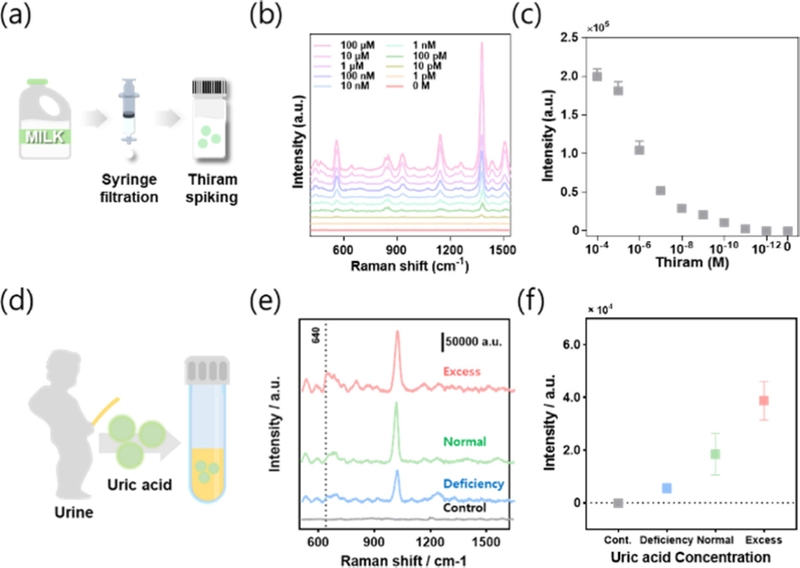
Application of hybrid nanostructure. (a) Schematic of the SERS sensor applied to detect thiram in milk using hybrid nanostructure. (b–c) SERS spectra and corresponding intensities of thiram at different concentrations in milk. (d) Schematic of the SERS sensor preparation for detecting uric acid in artificial urine using hybrid nanostructure. (e–f) SERS spectra and corresponding intensities of uric acid at different concentrations in artificial urine.
This promoted the oxidation of the SERS nanostructures, and an oxidation-resistant hybrid structure was employed to enhance the stability. The sensor demonstrated sensitive detection of uric acid in artificial urine, covering a range from 30 μM (deficient levels) to 300 μM (excessive levels), confirming its utility for sensitive and reliable SERS-based biosensing (Fig. 7 (d–f)).
5. CONCLUSION
In this review, we introduced advanced SERS nanopillar substrates, focusing on functional enhancements based on EC-fabricated nanopillars for high-sensitivity SERS analysis. In addition, we discuss the detection of biomarkers and environmental toxicants using various SERS structures.
EC nanopillars offer significant advantages over conventional MEMS processes, including simpler fabrication procedures and lower costs. These structures facilitated the formation of nanogaps and exhibited enhanced SERS effects, enabling their application in diverse detection fields. With ongoing advancements, EC SERS nanoelectrodes are expected to play an increasingly critical role in optical sensor analysis. Innovations in EC nanostructure growth methods (for example, cyclic voltammetry and pulse voltammetry) could further improve these platforms. Furthermore, additional particle growth on high-sensitivity hybrid nanostructures can further amplify the SERS effects.
In conclusion, the SERS substrates based on EC-fabricated nanopillars demonstrate outstanding SERS performance, ease of fabrication, and cost-effectiveness. These attributes render them highly promising for a broad range of SERS sensor applications.
Acknowledgments
This study was supported by the National Research Foundation of South Korea (NRF) under Grant Nos. NRF-2023R1A2C2004964 and RS-2024-00438542.
References
-
J.H. Cho, G. Bae, K.-S. An, Surface-Engineered Graphene surface-enhanced Raman scattering Platform with Machine-learning Enabled Classification of Mixed Analytes, J. Sens. Sci. Technol. 33 (2024) 139–146.
[https://doi.org/10.46670/JSST.2024.33.3.139]

-
P.P. Mandrekar, M. Lee, T.-S. Kim, D. Yang, Sensing and Identification of Health Hazardous Molecular Components using Surface-Enhanced Raman Spectroscopy: A Mini Review, J. Sens. Sci. Technol. 32 (2023) 259–266.
[https://doi.org/10.46670/JSST.2023.32.5.259]

-
H. Park, K. Chai, E. Park, W. Kim, G. Kim, J. Park, et al., Optimization of Paper-Based Alveolar-Mimicking SERS Sensor for High-Sensitivity Detection of Antifungal Agent, Biosensors 14 (2024) 566.
[https://doi.org/10.3390/bios14120566]

-
S. Boyd, M.F. Bertino, S.J. Seashols, Raman spectroscopy of blood samples for forensic applications, Forensic Sci. Int. 208 (2011) 124–128.
[https://doi.org/10.1016/j.forsciint.2010.11.012]

-
P. Colomban, On?site Raman study of artwork: Procedure and illustrative examples, J. Raman Spectrosc. 49 (2018) 921–934.
[https://doi.org/10.1002/jrs.5311]

-
T. Itoh, M. Procházka, Z.-C. Dong, W. Ji, Y.S. Yamamoto, Y. Zhang, et al., Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications, Chem. Rev. 123 (2023) 1552–1634.
[https://doi.org/10.1021/acs.chemrev.2c00316]

-
B. Sharma, R.R. Frontiera, A.-I. Henry, E. Ringe, R.P. Van Duyne, SERS: Materials, applications, and the future, Mater. Today 15 (2012) 16–25.
[https://doi.org/10.1016/S1369-7021(12)70017-2]

-
S.-Y. Ding, E.-M. You, Z.-Q. Tian, M. Moskovits, Electromagnetic theories of surface-enhanced Raman spectroscopy, Chem. Soc. Rev. 46 (2017) 4042–4076.
[https://doi.org/10.1039/C7CS00238F]

-
H. Ma, S.-Q. Pan, W.-L. Wang, X. Yue, X.-H. Xi, S. Yan, et al., Surface-Enhanced Raman Spectroscopy: Current Understanding, Challenges, and Opportunities, ACS Nano 18 (2024) 14000–14019.
[https://doi.org/10.1021/acsnano.4c02670]

-
Z. Zeng, Y. Liu, J. Wei, Recent advances in surface-enhanced raman spectroscopy (SERS): Finite-difference time-domain (FDTD) method for SERS and sensing applications, Trac-Trends Anal. Chem. 75 (2016) 162–173.
[https://doi.org/10.1016/j.trac.2015.06.009]

-
I. Chaudhry, G. Hu, H. Ye, L. Jensen, Toward modeling the complexity of the chemical mechanism in SERS, ACS Nano 18 (2024) 20835–20850.
[https://doi.org/10.1021/acsnano.4c07198]

-
Y. Liu, M. Kim, S.H. Cho, Y.S. Jung, Vertically aligned nanostructures for a reliable and ultrasensitive SERS-active platform: Fabrication and engineering strategies, Nano Today 37 (2021) 101063.
[https://doi.org/10.1016/j.nantod.2020.101063]

-
F. Usman, K.H. Ghazali, Y.W. Fen, F. Meriaudeau, R. Jose, Biosensing through surface enhanced Raman spectroscopy: a review on the role of plasmonic nanoparticle-polymer composites, Eur. Polym. J. 195 (2023) 112250.
[https://doi.org/10.1016/j.eurpolymj.2023.112250]

-
Z. Xu, A.M. Erinomo, Z. Dan, F. Qin, H. Chang, Flexible SERS substrates with gradient porous Cu structure dealloying from the thermal diffusion couples of Al/Cu stacking foils, Chem. Eng. J. 490 (2024) 151871.
[https://doi.org/10.1016/j.cej.2024.151871]

-
O. Guselnikova, H. Lim, H.J. Kim, S.H. Kim, A. Gorbunova, M. Eguchi, et al., New trends in nanoarchitectured SERS substrates: nanospaces, 2D materials, and organic heterostructures, Small 18 (2022) 2107182.
[https://doi.org/10.1002/smll.202107182]

-
X. Fu, H. Wu, Z. Liu, P. Wang, J. Rong, F. Fu, Z. Lin, Y. Dong, MoS2 Nanosheets as Substrates for SERS-Based Sensing, ACS Appl. Nano Mater. 7 (2024) 3988–3996.
[https://doi.org/10.1021/acsanm.3c05606]

-
W. Kim, W. Kim, H. Park, J. Hong, W. Lee, J. Park, Ultrasensitive Cd2+ detection based on biomimetic magneto-Au nano-urchin SERS chip fabricated using a 3D printed magnetic mold, Spectrochim. Acta A Mol. Biomol. Spectrosc. 304 (2024) 123427.
[https://doi.org/10.1016/j.saa.2023.123427]

-
D. Bang, Y.W. Chang, J. Park, T. Lee, J. Park, J.-S. Yeo, et al., One-step electrochemical fabrication of vertically self-organized silver nanograss, J. Mater. Chem. C 1 (2013) 4851–4857.
[https://doi.org/10.1039/c3ta01278f]

-
Q. Chen, L. Zhao, H. Liu, Q. Ding, C. Jia, S. Liao, et al., Nanoporous silver nanorods as surface-enhanced Raman scattering substrates, Biosens. Bioelectron. 202 (2022) 114004.
[https://doi.org/10.1016/j.bios.2022.114004]

-
L. Zhang, Y. Guo, R. Hao, Y. Shi, H. You, H. Nan, et al., Ultra-rapid and highly efficient enrichment of organic pollutants via magnetic mesoporous nanosponge for ultrasensitive nanosensors, Nat. Commun. 12 (2021) 6849.
[https://doi.org/10.1038/s41467-021-27100-2]

-
O. Guselnikova, A. Trelin, Y. Kang, P. Postnikov, M. Kobashi, A. Suzuki, et al., Pretreatment-free SERS sensing of microplastics using a self-attention-based neural network on hierarchically porous Ag foams, Nat. Commun. 15 (2024) 4351.
[https://doi.org/10.1038/s41467-024-48148-w]

-
W. Lee, B.-H. Kang, H. Yang, M. Park, J.H. Kwak, T. Chung, et al., Spread spectrum SERS allows label-free detection of attomolar neurotransmitters, Nat. Commun. 12 (2021) 159.
[https://doi.org/10.1038/s41467-020-20413-8]

-
J.-A. Huang, M.Z. Mousavi, Y. Zhao, A. Hubarevich, F. Omeis, G. Giovannini, et al., SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping, Nat. Commun. 10 (2019) 5321.
[https://doi.org/10.1038/s41467-019-13242-x]

-
H.J. Kim, B. Kim, D. Lee, B.-H. Lee, C. Cho, Improvement of surface-enhanced raman spectroscopy response characteristics of nanoporous ag metal thin film with surface texture structures, J. Sens. Sci. Technol. 29 (2020) 255–260.
[https://doi.org/10.46670/JSST.2020.29.4.255]

-
M. Kim, D. Park, J. Park, J. Park, Bio-Inspired Molecularly Imprinted Polymer Electrochemical Sensor for Cortisol Detection Based on O-Phenylenediamine Optimization, Biomimetics 8 (2023) 282.
[https://doi.org/10.3390/biomimetics8030282]

-
N. Elgrishi, K.J. Rountree, B.D. McCarthy, E.S. Rountree, T.T. Eisenhart, J.L. Dempsey, A practical beginner’s guide to cyclic voltammetry, J. Chem. Educ. 95 (2018) 197–206.
[https://doi.org/10.1021/acs.jchemed.7b00361]

-
H. Park, J. Park, G. Lee, W. Kim, J. Park, Detection of chlorpyrifos using bio-inspired silver nanograss, Materials 15 (2022) 3454.
[https://doi.org/10.3390/ma15103454]

-
W. Kim, J. Park, W. Kim, S. Jo, M. Kim, C. Kim, et al., Bio-inspired Ag nanovilli-based sandwich-type SERS aptasensor for ultrasensitive and selective detection of 25-hydroxy vitamin D3, Biosens. Bioelectron. 188 (2021) 113341.
[https://doi.org/10.1016/j.bios.2021.113341]

-
M.F. Holick, Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease, Am. J. Clin. Nutr. 80 (2004) 1678S–1688S.
[https://doi.org/10.1093/ajcn/80.6.1678S]

-
H.A. Weiler, A. Bielecki, W. Fu, I. Demonty, S.P. Brooks, Cholesterol Interference in the Assessment of Vitamin D Status: A Canadian Health Measures Survey Biobank Project, J. Nutr. 154 (2024) 1676–1685.
[https://doi.org/10.1016/j.tjnut.2024.04.003]

-
H. Park, J. Park, W. Kim, W. Kim, J. Park, Ultra-sensitive SERS detection of perfluorooctanoic acid based on selfassembled p-phenylenediamine nanoparticle complex, J. Hazard. Mater. 453 (2023) 131384.
[https://doi.org/10.1016/j.jhazmat.2023.131384]

-
Y. Samet, L. Agengui, R. Abdelhédi, Electrochemical degradation of chlorpyrifos pesticide in aqueous solutions by anodic oxidation at boron-doped diamond electrodes, Chem. Eng. J. 161 (2010) 167–172.
[https://doi.org/10.1016/j.cej.2010.04.060]

-
S.A. Maggio, P.K. Janney, J.J. Jenkins, Neurotoxicity of chlorpyrifos and chlorpyrifos-oxon to Daphnia magna, Chemosphere 276 (2021) 130120.
[https://doi.org/10.1016/j.chemosphere.2021.130120]

-
J.J. Calvillo Solís, C. Sandoval-Pauker, D. Bai, S. Yin, T.P. Senftle, D. Villagrán, Electrochemical reduction of perfluorooctanoic acid (PFOA): an experimental and theoretical approach, J. Am. Chem. Soc. 146 (2024) 10687–10698.
[https://doi.org/10.1021/jacs.4c00443]

-
M. Wu, R. Sun, M. Wang, H. Liang, S. Ma, T. Han, X. Xia, J. Ma, L. Tang, Y. Sun, Analysis of perfluorinated compounds in human serum from the general population in Shanghai by liquid chromatography-tandem mass spectrometry (LC-MS/MS), Chemosphere, 168 (20117) 100–105.
[https://doi.org/10.1016/j.chemosphere.2016.09.161]

-
J. Park, K. Chai, W. Kim, T. Yoon, H. Park, W. Kim, et al., Highly enhanced Hg2+ detection using optimized DNA and a double coffee ring effect-based SERS map, Biosens. Bioelectron. 264 (2024) 116646.
[https://doi.org/10.1016/j.bios.2024.116646]

-
V. Reyes-Cruz, C. Ponce-de-León, I. González, M. Oropeza, Electrochemical deposition of silver and gold from cyanide leaching solutions, Hydrometallurgy, 65 (2002) 187–203.
[https://doi.org/10.1016/S0304-386X(02)00083-X]

-
W. Kim, W. Lee, H. Park, J. Park, W. Kim, B. Kang, et al., Biomimetic nano-pine-pollen structure-based surface-enhanced raman spectroscopy sensing platform for the hypersensitive detection of toxicants: Cadmium and amyloid, ACS Sustain. Chem. Eng. 10 (2022) 3180–3190.
[https://doi.org/10.1021/acssuschemeng.1c07117]

-
A.K. James, S. Nehzati, N.V. Dolgova, D. Sokaras, T. Kroll, K. Eto, et al., Rethinking the Minamata tragedy: what mercury species was really responsible?, Environ. Sci. Technol. 54 (2020) 2726–2733.
[https://doi.org/10.1021/acs.est.9b06253]

-
S. Singh, A. Numan, Y. Zhan, V. Singh, T. Van Hung, N.D. Nam, A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework, J. Hazard. Mater. 399 (2020) 123042.
[https://doi.org/10.1016/j.jhazmat.2020.123042]

-
Y. Miyake, H. Togashi, M. Tashiro, H. Yamaguchi, S. Oda, M. Kudo, et al., MercuryII-mediated formation of thymine− HgII− thymine base pairs in DNA duplexes, J. Am. Chem. Soc. 128 (2006) 2172–2173.
[https://doi.org/10.1021/ja056354d]

-
W. Kim, G. Kim, H. Park, K. Chai, J. Park, J. Park, Detecting and tracking thiram in leakage pathways using bioinspired nanograss with thuja fruit-like nanoparticles, Sens. Actuator B Chem. 406 (2024) 135405.
[https://doi.org/10.1016/j.snb.2024.135405]

-
H. Park, K. Chai, W. Kim, J. Park, W. Lee, J. Park, Asterias forbesi-Inspired SERS Substrates for Wide-Range Detection of Uric Acid, Biosensors, 14 (2023) 8.
[https://doi.org/10.3390/bios14010008]

-
A. Hussain, D.-W. Sun, H. Pu, Bimetallic core shelled nanoparticles (Au@ AgNPs) for rapid detection of thiram and dicyandiamide contaminants in liquid milk using SERS, Food Chem. 317 (2020) 126429.
[https://doi.org/10.1016/j.foodchem.2020.126429]

-
E. Ozanturk, Z. Ucar, Y. Varol, H. Koca, A. Demir, D. Kalenci, et al., Urinary uric acid excretion as an indicator of severe hypoxia and mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease, Rev. Port. Pneumol. 22 (2016) 18–26.
[https://doi.org/10.1016/j.rppnen.2015.06.002]


Kyunghwan Chai is currently doing his Ph.D. course in Department of Biopharmaceutical Convergence, Sungkyunkwan University, South Korea. His main research focuses on detecting biomaterials using Raman spectroscopy and plasmonic system. Also, he is interested in the development of optical method based SERS substrate platforms.

Hyunjun Park is currently working as a biotechnology researcher (postdoctoral fellow) with a Ph.D. in Biomechatronics at Sungkyunkwan University. His main research focuses on manufacturing advanced SERS substrate for highly sensitive detection of environmental toxicants and biomarkers using Raman spectroscopy. He is also interested in SERS data analysis research using artificial intelligence

Jinsung Park is a professor of Department of Bio-Mechatronic Engineering, Sungkyunkwan University, South Korea. He received B.S. and Ph.D. from Department of Mechanical Engineering, Korea University in 2007 and 2013, respectively. He completed his postdoctoral fellow at Korea University in 2013. His research focuses on investigation of biomolecular interactions using atomic force microscopy, mechanical property of biomolecules and nanostructure, surface potential measurement for biomolecules and nanomaterials using Kelvin probe force microscopy, and environmental nano-toxic material detection using various sensing method. He is also interested in detecting toxic substances and biomolecules using electrochemical and SERS sensors.