Metal Oxide-based Electrochemical Non-enzymatic Glucose Biosensors: A Mini-Review
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Electrochemical glucose-sensing devices have advanced significantly in recent years, driven by the rising prevalence of diabetes mellitus, which is projected to become the seventh leading cause of death by 2030. The surge in the number of diabetes cases has accelerated the development of high-sensitivity and high-selectivity glucose biosensors. Traditional enzymatic glucose biosensors based on glucose oxidase or glucose dehydrogenase have evolved over multiple generations to overcome challenges, such as oxygen dependence, mediator toxicity, and enzyme instability. The latest fourth-generation glucose biosensors utilize metal-oxide-based catalysts instead of biological enzymes, offering enhanced stability, sensitivity, and cost-effectiveness. Nanoparticles (NPs) of metal oxides such as NiO, CuO, and Co3O4 exhibit high electrocatalytic activity and redox stability, making them promising materials for non-enzymatic glucose sensing. This review explores the recent advancements in metal oxide-based non-enzymatic glucose biosensors, particularly those employing NiO, CuO, and Co3O4 NPs, with a focus on electrocatalytic mechanisms, material innovations, and sensor fabrication strategies. Additionally, it highlights the current limitations and challenges of metal oxide-based glucose biosensors, while examining their potential applications in continuous glucose monitoring and wearable biosensors.
Keywords:
Metal oxide, Glucose, Electrochemical biosensor, Nanoparticles, Diabetes1. INTRODUCTION
Diabetes mellitus is a significant health concern affecting millions of people worldwide [1]. It is a chronic metabolic disorder characterized by abnormal blood sugar levels due to insulin dysfunction [2]. According to the World Health Organization (WHO) and International Diabetes Federation (IDF), approximately 9.3% of the global population (463 million people in 2019) [3] suffers from diabetes, leading to severe complications such as cardiovascular disease, kidney failure, stroke, eye and dental disorders, nerve damage, and limb amputation [1,4]. The prevalence of diabetes continues to increase, driven by factors such as unhealthy dietary habits, a sedentary lifestyle, and obesity. Projections by the IDF indicate that the global prevalence of diabetes will reach 10.2% (578 million cases) by 2030 and 10.9% (700 million cases) by 2045 [3]. Additionally, WHO predicted that diabetes will become the seventh leading cause of death by 2030 [5,6].
Diabetes arises from either a deficiency in insulin production (type 1 diabetes) or an impaired ability of the body to utilize insulin effectively (type 2 diabetes) [7]. Treatment typically involves insulin administration and lifestyle modifications to regulate blood sugar levels. However, glucose levels fluctuate throughout the day owing to factors such as food intake, physical activity, and medication. Regular glucose monitoring is vital to maintain blood glucose levels [8,9] within the optimal range, thereby reducing the risk of complications associated with hypoglycemia (low blood sugar levels) and hyperglycemia (high blood sugar levels). Regular glucose monitoring plays a crucial role in the management of diabetes and improvement of patient outcomes.
Several techniques for glucose measurement have been developed, including colorimetry [10,11], conductometry [12], fluorescence spectroscopy [13], and electrochemical sensing [14]. Among these methods, electrochemical sensing has been the leading technology in the global market for the past 40 years [15] owing to its exceptional sensitivity and selectivity for glucose detection. Additionally, its affordability and user-friendly operation make it highly accessible even to non-medical personnel, further contributing to its widespread adoption [16].
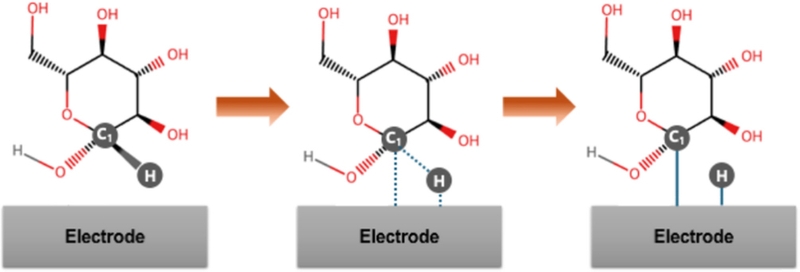
Enzymatic glucose biosensors can be divided into three primary generations (Fig. 1) [17]. The first-generation biosensor was based on Clark and Lyons’ concept [18]. Flavin adenine dinucleotide (FAD/FADH2) in glucose oxidase (GOx), a biological recognition element, is paired with dissolved oxygen in a blood sample as an electron acceptor to generate an electrochemical signal [19].
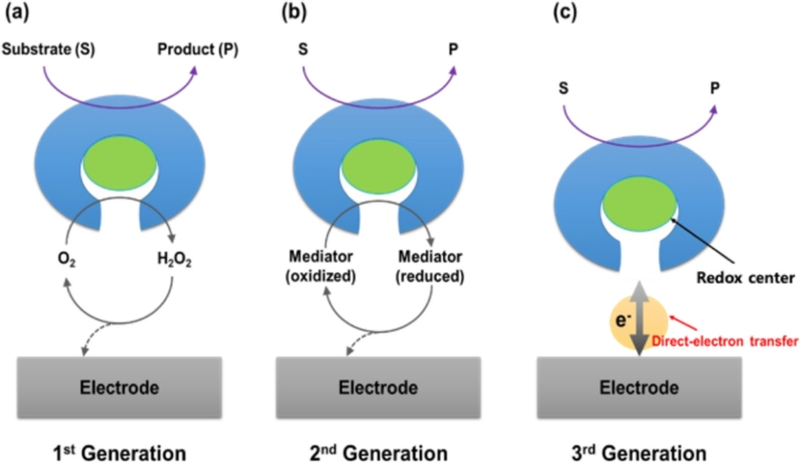
The development of electrochemical enzymatic biosensor. (a-c) Illustrative representations of first-, second-, and third-generation electrochemical enzymatic biosensors, respectively.
| (1) |
| (2) |
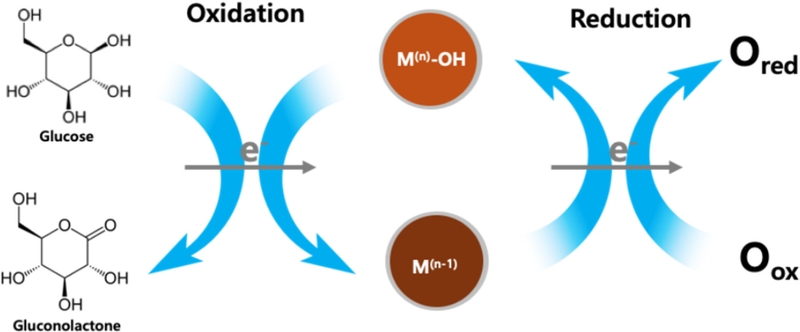
GOx catalyzes the oxidation of glucose (C6H12O6) to gluconolactone (C6H10O6), producing hydrogen peroxide (H2O2) as a byproduct. The generated H2O2 was oxidized at the electrode surface, producing an electrical current proportional to the glucose concentration. However, this system relies on dissolved free oxygen as a mediator, making the measurement dependent on the oxygen concentration in the sample [16].
Artificial redox mediators have been introduced to overcome the limitations of first-generation biosensors. These mediators [20], such as ferro/ferricyanide, hydroquinone, ferrocene, and osmium complexes, facilitate reversible redox reactions, enabling efficient electron transfer between the redox center of the enzyme and the electrode surface [21]. This process is illustrated by the following reactions, where M(ox) and M(red) represent the oxidized and reduced forms of the mediator, respectively [19].
| (3) |
| (4) |
| (5) |
Second-generation glucose biosensors have significantly improved glucose detection accuracy by minimizing oxygen dependency, enhancing electron transfer speed, and reducing interference due to the lower operating potential. These advancements have directly contributed to the development of commercial glucose test strips and portable glucometers that are now widely used in diabetes management.
The third-generation biosensor was developed to eliminate the need for toxic mediators by enabling direct electron transfer (DET) between the redox center of FAD/FADH2in GOx and the electrode surface [14]. This is illustrated by the following reaction [14].
| (6) |
| (7) |
However, achieving efficient DET remains a challenge because of the deeply embedded redox center (e.g., FAD/FADH2) of GOx within its nonconductive protein structure [22]. To enhance the DET efficiency, most studies have focused on nanostructured electrode surfaces [14,22], which improve electron transfer. However, these approaches often suffer from reproducibility issues during biosensor fabrication, which affect large-scale production and consistency.
Fourth-generation glucose biosensors represent the most recent advancements in glucose sensing technology [2,17]. This system employs metal-based catalysts instead of biological catalysts such as GOx or glucose dehydrogenase (GDH). Eliminating enzymatic components enhances stability and overcomes challenges such as enzyme degradation, denaturation, and sensitivity to environmental factors such as pH, temperature, and humidity [15,23]. In these sensors, the oxidation of glucose is facilitated by various electrocatalysts, including noble metals, metal oxides, alloys, and metal complexes. Among these, metal oxide-based glucose sensors have gained significant attention for non-enzymatic glucose detection owing to their high electrocatalytic activity, long-term redox stability, improved sensitivity, and cost-effectiveness [15,17,24].
2. Glucose oxidation mechanism on metal oxide-based electrode surface
Glucose oxidation in non-enzymatic sensors is widely explained by two models: the chemisorption model [25] and the incipient hydrous oxide adatom mediator (IHOAM) model.
According to the chemisorption model (Fig. 2), glucose oxidation begins when a glucose molecule approaches the electrode surface. The predominance of transition metal species in electrocatalysts arises because of their unpaired d-electrons and unfilled d-orbitals, which are available to form bonds with adsorbates (e.g., adsorption of the C1 carbon and its hydrogen atom onto the electrode surface) [25]. This adsorption is followed by C–H bond cleavage at the C1 position, initiating glucose oxidation.
The IHOAM model is a theoretical framework that describes the electrocatalytic behavior of metal oxides in non-enzymatic glucose sensing [26]. It explains how transition metal oxides (e.g., NiO, CuO, Co3O4, MnO2, ZnO) facilitate glucose oxidation through the formation and involvement of hydrous oxide layers [26]. Extensive research [2,15,17] suggests that when a metal oxide electrode is immersed in an alkaline electrolyte containing hydroxide ions (OH-), a thin layer of hydrous oxide (M–OH) forms on its surface. This M–OH layer serves as a key mediator, enhancing charge transfer and improving the glucose electrooxidation efficiency. During glucose oxidation, the hydrous oxide layer undergoes a redox cycle that alternates between the oxidized and reduced states. This continuous transition enables the metal oxide to reversibly shift between different oxidation states, thereby facilitating glucose oxidation, as shown in Fig. 3 [26], where M(n) and M(n-1) denote different oxidation states of the metal oxide.
3. Metal oxide-based non-enzymatic glucose sensors
3.1 Cobaltosic oxide-based glucose sensors
Cobalt oxide is widely used in non-enzymatic electrochemical glucose sensors. It exists in three well-known polymorphic forms: (1) cobaltous oxide (CoO), (2) cobaltic oxide (Co2O3), and (3) cobaltosic oxide (Co3O4) [17], each of which exhibits unique electrochemical properties suitable for glucose-sensing applications. Among them, Co3O4, also known as tricobalt tetroxide, is a mixed-valence transition metal oxide consisting of both Co2+ and Co3+ oxidation states. It is widely utilized owing to its exceptional electrocatalytic and redox properties, making it a highly valuable material for various applications, particularly in non-enzymatic glucose biosensors. In 2010, Ding et al. [28] reported electrospun Co3O4 nanofibers for non-enzymatic electrochemical detection of glucose. Their study revealed that no redox peaks were observed in a neutral electrolyte, whereas two distinct redox pairs were detected in an alkaline solution (Fig. 4 (c)) [28,29]. These redox reactions are described by the following equations [29,30]:
| (8) |
| (9) |
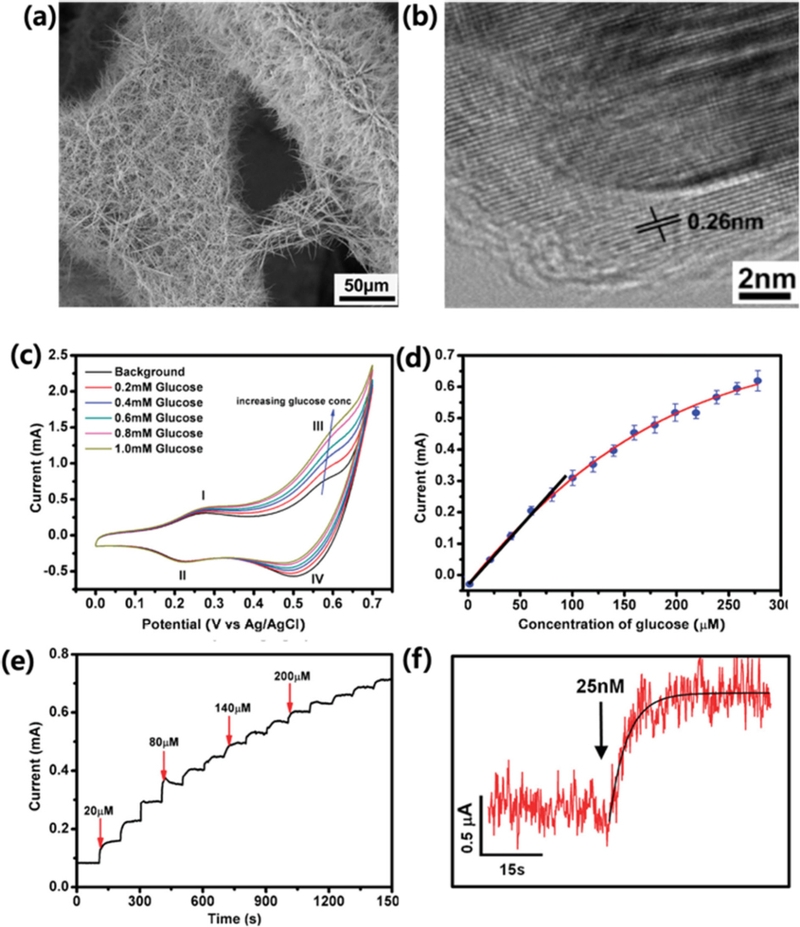
3D graphene/Co3O4 nanowire-based non-enzymatic glucose biosensors. A SEM images of (a) 3D graphene/Co3O4 nanowire composite. (b) A TEM image of Co3O4 nanowire grown on the surface of 3D graphene foam. (c) CV curves in the presence of various concentrations of glucose (0, 0.2, 0.4, 0.6, 0.8, and 1 mM), at the scan rate of 20 mV/s. (d) A calibration curve obtained from three different sensors, with a linear fitting at lower concentration range and an exponential fitting at higher concentration range. (e) Amperometric response of the composite electrode (holding at 0.58 V) upon addition of glucose to increasing concentrations. (f) Amperometric response to 25 nM glucose. Reprinted (adapted) with permission from ref [37]. Copyright (2012) American Chemical Society.
This suggests that the electrochemical redox reaction of Co3O4 requires OH- ions to proceed. Additionally, many studies [28,30,31] have proposed a glucose oxidation mechanism in which the CoO2/CoOOH redox pair plays a crucial role [32] in mediating glucose oxidation at approximately 0.6 V vs. Ag/AgCl (Fig. 4 (c)):
| (10) |
Later, Hou et al. [33] synthesized Co3O4 nanoparticles (NPs) (~20 nm in diameter) by annealing metal-organic framework (MOF) templates. MOFs are widely recognized as exceptional metal ion adsorbents because of their high surface areas, tunable pore structures, and abundant active sites [34]. Upon calcination, MOFs undergo thermal decomposition, leading to their transformation into nanostructured metal oxide NP with enhanced electrocatalytic activities [17]. The resulting biosensor exhibited a sensitivity of 520.7 μA/mM·cm2 and a limit of detection (LOD) of 0.13 μM. Similarly, Balouch et al. [31] developed Co3O4 nanoflowers for non-enzymatic glucose detection, achieving a remarkable sensitivity of 1618 μA/mM·cm2 with an LOD of 0.1 μM. This high sensitivity can be attributed to superior electrocatalytic activity of Co3O4, which arises from its large specific surface area and numerous active sites, significantly enhancing glucose oxidation efficiency [35,36].
In addition to morphology, the choice of substrate has also been found to play a crucial role in improving sensor sensitivity. To further enhance performance, researchers have widely explored hybrid structures of Co3O4 with conductive metals or nanocarbons. One such approach involved employing a highly conductive graphene layer as a template for Co3O4. A nanocarbon-Co3O4 hybrid structure [37] was fabricated via hydrothermal synthesis of Co3O4 on a CVD-grown three-dimensional (3D) graphene foam. In this structure, a 3D porous graphene skeleton was uniformly coated with Co3O4 nanowires (~200–300 nm in diameter) (Fig. 4 (a) and (b)). In agreement with Ding’s report [28], two significant pairs of redox peaks (I/II and III/IV) were observed because of the reversible transitions between Co3O4 and CoOOH (I/II) and between CoOOH and CoO2 (III/IV) (Fig. 4 (c)) [37]. Due to the synergistic effect of highly electrocatalytic Co3O4 nanowires on a conductive graphene framework, the system exhibited exceptional glucose sensitivity of 3.4 mA/mM·cm2 with an LOD of 25 nM (Fig. 4 (d) and (e)). Lang et al. [38] further advanced this concept by developing flexible microelectrodes with seamless nanoporous gold-cobalt oxide hybrid structures. In their design, a highly conductive solid gold microwire was electrochemically etched to create nanopores that increased the specific surface area. Co3O4 was then hydrothermally grown within these nanopore channels. Due to the synergistic effect of the high-surface, conductive gold skeleton and the electrocatalytic activity of Co3O4 NPs [38], the biosensor demonstrated an impressive sensitivity of 12.5 mA/mM·cm2, a rapid response time of less than 1 s, and an LOD of 5 nM.
3.2 Copper oxide-based glucose sensors
Owing to its non-toxic nature, high abundance, excellent electrochemical properties, and strong catalytic activity, copper oxide (CuO) has garnered significant attention as a promising catalyst for glucose oxidation in non-enzymatic electrochemical biosensors [17,32]. CuO synthesis methods, such as hydrothermal [39], sol-gel [40], and electrodeposition [41], are simple, scalable, and cost-effective compared with other metal-based glucose sensors. CuO can be engineered into various nanostructures (e.g., nanorods, nanowires, nanosheets, and NPs) to increase its active surface area and improve glucose interactions [2,39,40]. In addition, nanostructured CuO enhanced electron transport, leading to higher sensitivity and faster detection at lower overpotential [42].
When CuO is immersed in an alkaline electrolyte (e.g., a NaOH or KOH solution), it undergoes electrochemical oxidation to form Cu3+ species (e.g., CuOOH), which act as active redox mediators. CuOOH is the key catalytic species responsible for the oxidation of glucose. Glucose is subsequently oxidized by CuOOH, resulting in the formation of gluconolactone. Consequently, The fast regeneration of Cu2+ (e.g., CuO) occurs because the equilibrium in reactions (Eq. 12 and 13) lie towards the right, favoring the formation of CuO in an alkaline environment [43]. This rapid regeneration ensures a continuous catalytic cycle, maintaining the high electrocatalytic efficiency and stability of the sensor for glucose oxidation. The redox state change of Cu2+/Cu3+ during glucose oxidation contributes to the measurable electrochemical current, which is directly proportional to the glucose concentration. The overall reaction mechanism is described below [40,43,44]:
| (11) |
| (12) |
| (13) |
The morphologies and structures of Cu-based nanomaterials significantly influence their performance in glucose detection [2]. Sahoo et al. [39] produced faceted nanoribbons comprising a number of facets with widths of 50–100 nm by using hydrothermal methods. The amperometric oxidation current exhibited a linear increase with glucose concentration, demonstrating a sensitivity of 412 μA/mM·cm2 over the range of 0.05–3.5 mM and achieving a LOD of 58 μM.
Luo et al. [46] fabricated porous hierarchical CuO nanospheres by the calcination of a Cu-MOF. The MOF-driven CuO porous hierarchical nanospheres demonstrate a high sensitivity of 1.8 mA/mM·cm2 within a broad linear detection range of 0–6.535 mM, along with a LOD of 0.15 μM. Glucose detection was further evaluated in artificial saliva, which showed exceptional sensing capability within the low concentration range of 5 μM to 1.165 mM, highlighting its potential for noninvasive glucose monitoring. Electron transfer kinetics can be improved by incorporating CuO into conductive carbonaceous nanomaterials. Wang et al. [40] reported the facile and green synthesis of CuO-reduced graphene oxide (rGO) nanocomposites in a water-isopropanol solution. Because isopropanol acts as both a solvent and reductant in a graphene oxide (GO)/copper ion mixture, CuO NPs were successfully decorated on the graphene surface. Owing to the synergistic interaction between the high specific surface area and conductive nature of rGO and the rich active sites of CuO NPs that were directly anchored on the conductive nanocarbon, the fabricated CuO/rGO/GCE sensor lowered the activated potential to 0.4 V vs. Ag/AgCl and exhibited the a high sensitivity of 2.2 mA/mM·cm2 with a wide linear range from 0.4 μM to 12 mM for glucose. A laser-engraving technique was utilized for the fabrication of flexible CuO-based biosensors. As shown in Fig. 5, Li et al. [45] employed a two-step laser irradiation process on a polyimide (PI) surface. Initially, a Cu precursor solution was applied and subjected to laser-assisted Cu reduction to form a reduced Cu layer on the PI substrate. This was followed by a second laser exposure, which facilitated the formation of laser-engraved graphene (LEG) as a conductive electrode. To further enhance the catalytic performance, the copper (Cu) NP/LEG-based structure was subjected to O2 plasma treatment, converting Cu NPs into CuO NPs (Fig. 5 (b)). As the glucose concentration increased, the oxidation signal at ~0.6 V vs. Ag/AgCl increased (Fig. 5 (c)) as described in Eq. 11–13. The CuO/LEG sensor exhibits a high sensitivity of 619.43 μA/mM·cm2 in 0–3 mM glucose and 462.96 μA/mM·cm2 in 0–8 mM glucose (Fig. 5 (d) and (e)). The integration of CuO and laser-induced graphene (LIG) through direct laser-assisted writing results in a flexible sensor with excellent bending stability, retaining approximately 82% of its initial current after 100 bends [45]. The electrodeposition of CuO enhances the effective anchoring of catalytic CuO NPs onto the electrode surface, thereby improving the sensitivity [41]. Cyclic voltammetry (CV) or chronoamperometric deposition in an electrolyte containing copper ions enables the controlled formation of CuO or Cu2O NPs directly on the working electrode surface. Zhang et al. [41] utilized electrochemical deposition on conductive carbon cloth in a copper ion-containing electrolyte, followed by annealing at 260°C. The combination of high conductivity from the carbon cloth, large specific surface area from the CuO/Cu2O nanostructures, and enhanced electron transfer through the in-situ grown structure resulted in an electrode with high sensitivity (6.5 mA/mM·cm2) and an ultra-low LOD (60 nM).
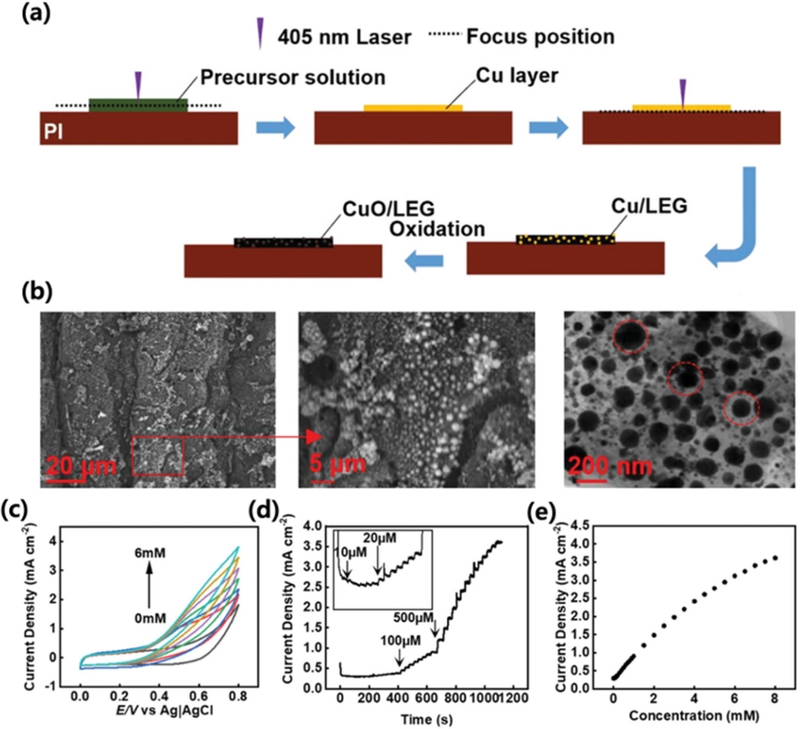
CuO/LEG (laser-engraved graphene) electrode for non-enzymatic glucose sensing. (a) Schematic diagram of preparation of CuO/LEG electrode by laser direct writing. (b) SEM and TEM images of CuO/LEG nanocomposite. Red circles in a TEM image indicate the CuO NPs embedded in LEG matrix (c) CV curves of the CuO/LEG electrode in the presence of 0–6 mM glucose in a 0.1 m NaOH solution with constant scan rate of 50 mV/s. (d) Amperometric response of the CuO/LEG sensor on the successive addition of glucose. (e) Dependence of the current density of the sensor on glucose concentration from 0 to 8 mM. Reprinted (adapted) with permission from ref [45], copyright (2013) Advanced Sensor Research, Creative Commons Attribution-Non Commercial License (CC-BY-NC).
3.3 Nickel oxide-based glucose sensors
Nickel (II) oxide (NiO) is considered one of the most promising materials for non-enzymatic glucose biosensors because of its low toxicity, natural abundance, affordability, excellent catalytic properties, and high electrochemical stability [2,5,15]. Since Fleischmann et al. [47] first demonstrated that nickel exhibits excellent electrocatalytic activity for the oxidation of organic compounds under alkaline conditions, extensive research has been conducted on Ni-based materials for non-enzymatic glucose biosensing. NiO benefits from a highly reversible Ni2+/Ni3+ redox couple [48], which enhances glucose oxidation efficiency. Similar to the CuO-based glucose sensors, NiO forms NiOOH under alkaline conditions. Thus, NiOOH acts as a strong electrocatalyst for glucose oxidation as described below [32,47,48]:
| (14) |
| (15) |
| (16) |
A variety of studies have employed hydrothermal methods to produce NiO NP catalysts, followed by calcination, to obtain glucose-sensing performance. Wang et al. [49] hydrothermally prepared NiO nanosheets and loaded them onto a glass-carbon electrode. Their study revealed that NiOOH formation plays a crucial role in glucose oxidation [49]. The two-dimensional morphology of NiO nanosheets, featuring a large surface area, contributes to their high sensitivity of 400 μA/mM·cm2 and a LOD of 1 μM.
As shown in Fig. 6, He et al. [50] reported the synthesis of a cubic NiO hollow porous architecture (NiO HPA) using the coordinating etching and precipitation (CEP) method, followed by post-calcination. The TEM and SEM images revealed a hollow cubic structure composed of NiO, highlighting its well-defined morphology and porous architecture (Fig. 6 (a) and (b)). When utilized for glucose detection, the NiO HPA electrode demonstrated remarkable electrocatalytic performance, achieving high sensitivity (1323 μA/mM·cm2) and a low LOD (0.32 μM). The exceptional catalytic activity at the potential of 0.37 and 0.5 V vs. Ag/AgCl (Fig. 6 (c)) is attributed to its large specific surface area, well-ordered diffusion channels, and enhanced electron transfer rate, all of which stem from its unique hollow porous structure [50]. In terms of selectivity, the NiO HPA electrode exhibited minimal interference, with less than 8.7% signal deviation from the common interfering species (Fig. 6 (d)). Additionally, it retained 89.02% of its initial response after 30 d, highlighting its excellent long-term stability.
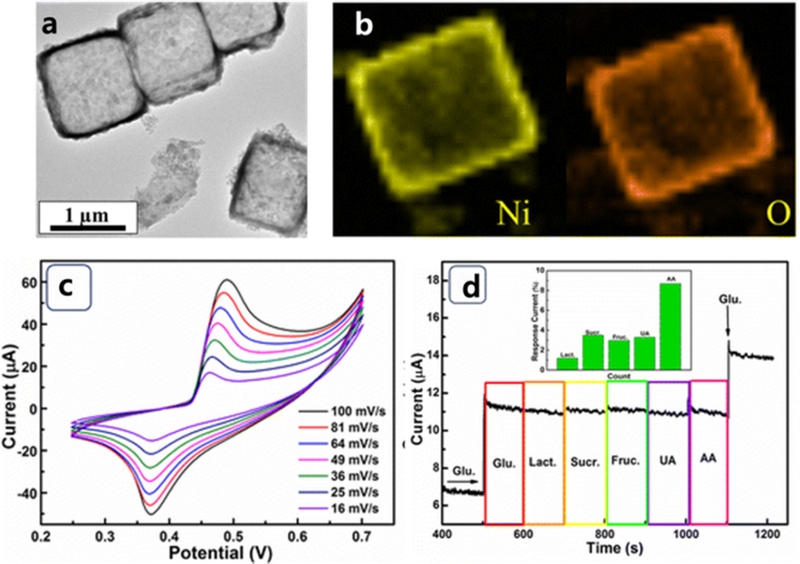
NiO hollow porous cubes for non-enzymatic glucose biosensor. (a) A TEM image of NiO HPA. (b) EDX mapping images of a NiO HPA cube. (c) CVs of NiO HPA electrode at various scan rates in 0.1 M NaOH with 1 mM glucose. (d) The current response of NiO HPA electrode to sequential addition of 50 μM glucose and 5 μM interfering species at an applied potential of 0.6 V. Inset is the statistical data of the interference current. Reprinted with permission from ref [50], copyright (2017) Nanoscale Research Letters, Creative Commons Attribution 4.0 International License (CC-BY-4.0).
A novel laser-induced oxidation method was developed for the in-situ formation of nanoporous NiO structures directly on a nickel surface, enabling a highly sensitive non-enzymatic glucose sensor (Fig. 7) [51]. The charge transfer resistance significantly decreased from 759.9 kΩ to 5.1 kΩ after laser-induced oxidation, attributed to the increased surface area and enhanced catalytic activity resulting from the laser-engraving process. The non-enzymatic glucose electro-oxidation exhibited a substantial improvement in a laser-irradiated Ni electrode, compared to a pristine Ni electrode, achieving a linear sensitivity of 5.22 mA/mM·cm2 and a LOD of 3.3 μM. Wang et al. [52] developed a 3D NiO-Au heterostructure using a facile annealing and sputtering method. In this design, Au NPs (~20 nm in size) were aggregated into island-like structures on the surface of the NiO nanowrinkles, facilitating the formation of numerous Schottky interfaces while partially exposing the NiO. The resulting biosensor demonstrated high sensitivity (6.3 mA/mM·cm2) and a LOD of 1.2 μM. The enhanced sensitivity was attributed to glucose oxidation at the Au/NiO interface and the presence of multiple Schottky barriers, which significantly improved the electron transfer efficiency [52]. NiO NPs can also be incorporated into conducting polymers and carbonaceous materials. Kailasa et al. [53] synthesized a NiO NPs@polyaniline (PANI) composite by polymerizing conducting PANI along NiO NPs, resulting in the encapsulation of NiO within the conductive polymer matrix. The NiO NPs@PANI structure significantly enhanced glucose electrooxidation because of its large surface area, which facilitated efficient electron transfer while promoting the adsorption and diffusion of glucose molecules. Additionally, the incorporation of NiO NPs introduced more active sites, allowing for greater electron absorption and transfer to the PANI surface, thereby improving the kinetics of glucose oxidation. This amperometric non-enzymatic glucose sensor demonstrated high sensitivity (5.6 mA/mM·cm2) and a LOD of 0.06 μM. The electrochemical stability of the NiO-NP-decorated PANI nanosheets was evaluated by monitoring the time vs. current response over 60 d in the presence of 1 mM glucose in 0.1 M NaOH (alkaline solution) at 0.78 V of Ag/AgCl. The sensor retained 91.1% of its initial sensitivity by the 60th day, indicating its excellent long-term stability.
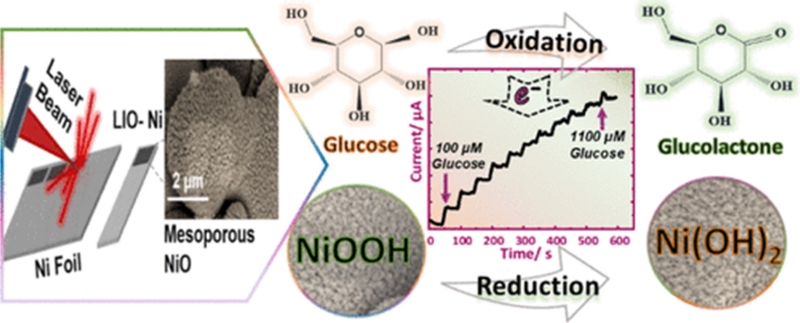
Schematic illustration of laser-induced mesoporous NiO fabrication and reaction mechanism with glucose. Reprinted (adapted) with permission from [51]. Copyright (2020) American Chemical Society.
A similar approach was applied to carbon-based materials, in which a nickel precursor was mixed with GO, followed by hydrothermal reduction and calcination (Fig. 8) [54]. NiO grown in situ on rGO (Fig. 8 (a)) demonstrated a significantly enhanced catalytic activity for glucose oxidation compared to bare NiO. This improvement is primarily attributed to the direct templating and growth of NiO NPs on the conductive rGO surface, which facilitates more efficient electron transfer and catalytic performance. The sensor demonstrated a sensitivity of 666 μA/mM·cm2 with a LOD of 5 μM (Fig. 8 (c)). The sensor exhibited almost no response to interfering substances, demonstrating excellent selectivity while showing a significant current increase in the presence of glucose, confirming its high specificity for glucose detection (Fig. 8 (d)). The long-term stability of the NiO/rGO sensor was assessed by tracking its amperometric response to 0.2 mM glucose at regular intervals over 18 d. The sensor retained more than 88% of its initial sensitivity, demonstrating exceptional durability and chemical resistance, making it a highly stable NiO-based glucose sensor suitable for extended use [54].
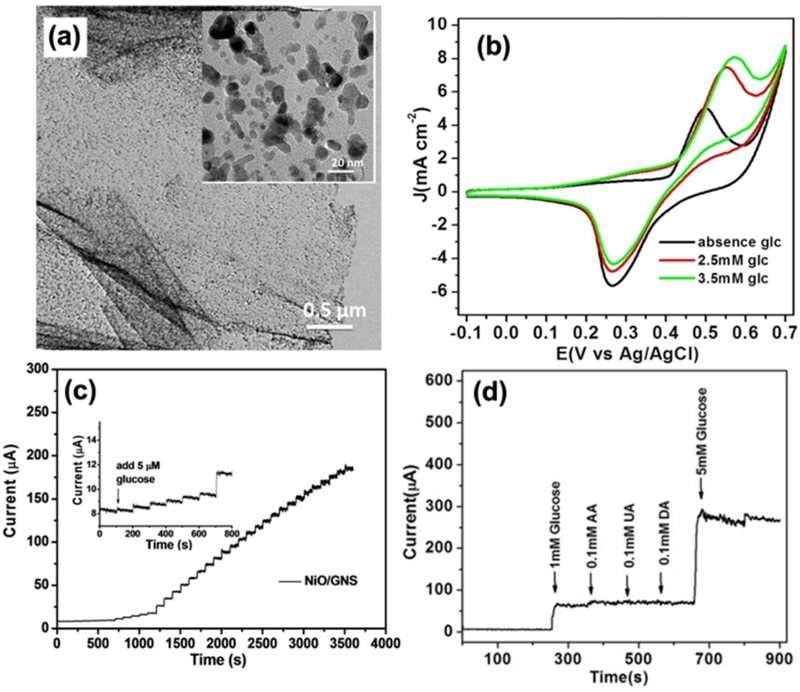
NiO/Graphene nanosheet (GNS)-based non-enzymatic glucose biosensor. (a) A TEM image of NiO/GNS (inset: A high-resolution TEM image showing the templated NiO NP). (b) CVs of the NiO/GNS in 0.1 M NaOH in the absence and presence of glucose at a scan rate 10 mV/s. (c) Amperometric response of the NiO/GNS to successive addition of glucose at 0.5 V; inset: amperometric response to 5.0 μM glucose. (d) Amperometric response of the NiO/GNS to successive addition of 0.1 mM AA, 0.1 mM UA, 0.1 mM DA to glucose at an applied potential of 0.50 V. Reprinted with permission from ref [54], copyright (2017) Scientific reports, Creative Commons Attribution 4.0 International License (CC-BY-4.0).
3.4 Multimetallic oxide-based glucose sensors
Multi-metallic oxide glucose biosensors, which incorporate two or more metal oxides (NiO, CuO, Co3O4, ZnO, Fe2O3, MnO2, etc.), have emerged as high-performance non-enzymatic glucose sensors due to their synergistic catalytic activity, enhanced electron transfer, and improved stability [2,17]. Multimetallic oxides provide more active redox centers [55], facilitating efficient glucose oxidation. Synergistic electron transfer between different metal oxides boosts charge transport and reaction kinetics [56].
Lotfi et al. [55] synthesized a NiO-CuO hybrid decorated on graphitic carbon nitride (g-C3N4). The g-C3N4 precursor was first mixed with nickel and copper precursors in an alkaline medium, followed by a metal ion reduction process. The resulting precipitate was calcined at 300°C to obtain the final composite. Compared to NiO@g-C3N4 and CuO@g-C3N4, the hybrid material exhibited a lower charge transfer resistance (Rct), indicating improved electron transport properties. The incorporation of binary metal oxides (NiO and CuO) into g-C3N4 significantly enhanced its conductivity as a semiconductor, reducing electron transfer resistance and improving overall electrocatalytic efficiency [55]. The synergistic effect of CuO and NiO promoted the electrocatalytic activity of the resulting electrode towards the oxidation of glucose because they provided more active sites for contact with glucose. The sensitivity was estimated to be 362 μA/mM·cm2. As shown in Fig. 9, Archana et al. [56] fabricated a hierarchical CuO/NiO-carbon nanocomposite (CuO/NiO-C) synthesized from a Cu/Ni-MOF precursor on cello tapes (CT). The study highlighted that the synergistic interaction between NiO and CuO in the CuO/NiO-C composite, along with the hierarchical porous structure formed during the annealing process, created abundant active sites for analyte accommodation (Fig. 9 (b–d)) [56]. This structural design significantly enhanced the interfacial contact between the analyte and the electrode, facilitating efficient glucose oxidation (Fig. 9 (f)). The sensor demonstrated a sensitivity of 586 μA/mM·cm2 with a LOD of 37 nM, confirming its high electrocatalytic performance. Owing to its integration on flexible CT, the sensor exhibited excellent stability and retained its performance even after exposure to various degrees of bending (Fig. 9 (f)).
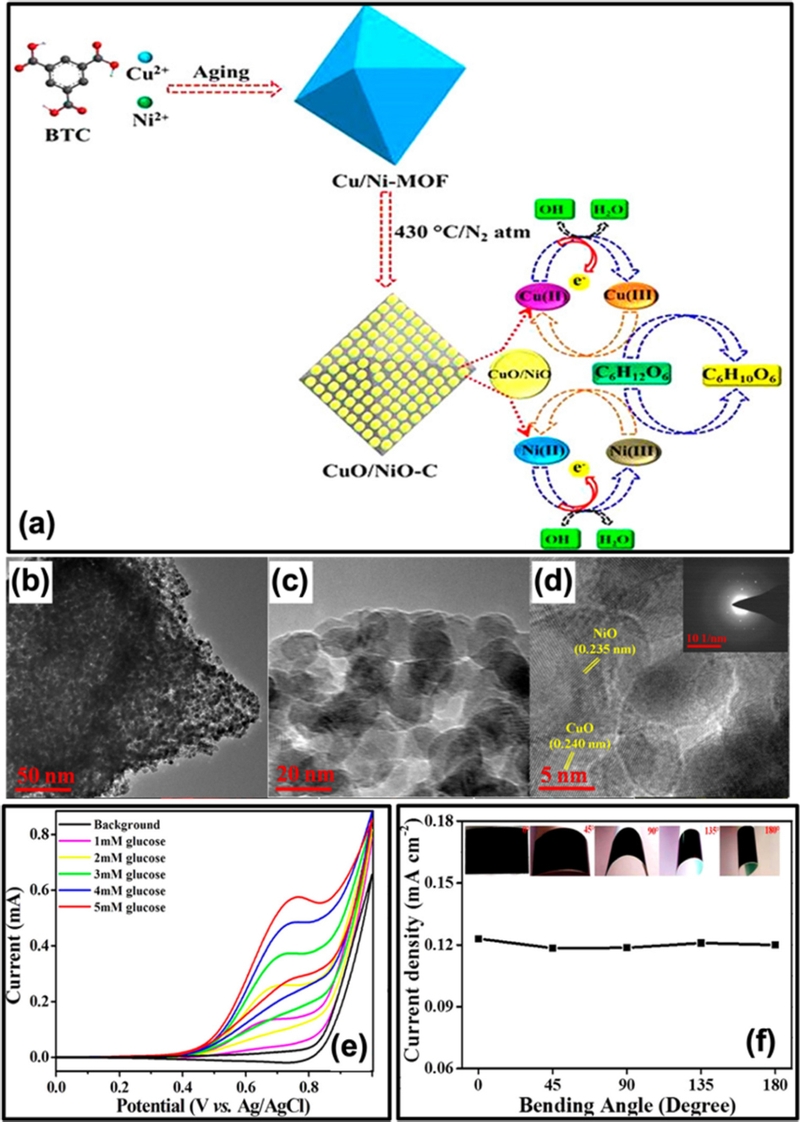
CuO/NiO-based non-enzymatic biosensor. (a) Schematic illustration describing the synthetic procedures and glucose electrooxidation mechanism at CuO/NiO-C/CT. (b–d) TEM images of CuO/NiO-C. (e) CVs of CuO/NiO-C/CT with the different concentration of glucose in 0.1 M NaOH at 100 mV/s. (f) Current density as a function of different bending angles of CuO/NiO-C/CT (Inset images showing the digital photographs of CuO/NiO-C/CT at different bending angles). Reprinted (adapted) with permission from [56]. Copyright (2019) American Chemical Society.
Li et al. [57] fabricated polyvinylpyrrolidone (PVP) nanofibers doped with copper and cobalt ions using an electrospray technique. The resulting metal-ion-doped PVP fibers were calcined to form Cu/Co-doped carbon nanofibers (CuCo–CFs). The superior glucose-sensing performance of the CuCo–CFs network was attributed to its well-developed conductive 3D net-like nanofilm structure and the synergistic effect of the Co(III)/Co(IV) and Cu(II)/Cu(III) redox couples, which enhanced the electrocatalytic activity. The sensor demonstrated a sensitivity of 507 μA/mM·cm2 in human serum samples, confirming its potential for biomedical glucose detection. Another study [58] reported a hierarchical Co3O4/CuO nanorod array on carbon cloth, fabricated via magnetron sputtering of a copper layer, followed by anodic oxidation to form Cu(OH)2. The electrode was then treated with cobalt ions and a reducing agent, followed by annealing, forming the final Co3O4/CuO structure. TEM analysis showed CuO nanorods encapsulated by interconnected Co3O4 nanoflakes, forming a core-shell structure. The sensor exhibited high sensitivity (5.405 mA/mM·cm2) and a low LOD (0.38 μM), attributed to: (1) Co3O4 enhancing electron transport due to large surface and effective interconnection to the CuO nanorod, (2) abundant electroactive sites of Co3O4/CuO for glucose oxidation, and (3) direct binder-free integration onto carbon cloth for improved conductivity. Xue et al. [59] developed graphenewrapped porous Co3O4/NiCo2O4 double-shelled nanocages (DSNCs) for glucose oxidation. The synthesis involved immersing ZIF-67 in a nickel ion solution, which allowed the nickel precursors to be embedded into the ZIF-67 structure. This was followed by calcination, forming Co3O4/NiCo2O4 DSNCs. The resulting nanocages were then dispersed in a GO solution and subjected to hydrothermal reduction, yielding Co3O4/NiCo2O4 DSNC@rGO. The incorporation of graphene significantly enhanced the electrocatalytic activity of the porous Co3O4/NiCo2O4 DSNCs due to its high specific surface area and excellent electrical conductivity. The sensor demonstrated a LOD of 0.384 μM and a sensitivity of 0.304 mA/mM·cm2. Its structural design effectively enhanced the electron transfer efficiency, promoting direct interaction between the glucose molecules and the electrode surface, which significantly improved the glucose oxidation performance.
3.5 Limitations and challenges of metal oxide-based glucose biosensor
Despite their potential, metal oxide-based glucose biosensors face several challenges that affect their practicality, stability, and scalability [60]. A major limitation of metal oxide-based glucose biosensors is their dependence on strong alkaline electrolytes, such as NaOH or KOH [2,60], which are essential for efficient glucose oxidation. Studies have demonstrated that the sensor sensitivity decreases as the electrolyte pH approaches physiological conditions (e.g., pH 7.2). This alkaline requirement presents biocompatibility challenges, making these sensors unsuitable for in vivo applications and limiting their integration into continuous glucose monitoring systems (CGMS) [61]. Another major drawback is the high-temperature calcination required for the synthesis of metal oxide nanostructures. This process, performed at temperatures ranging from 300 to 600°C [32], is energy-intensive, increases fabrication costs, and limits the use of flexible or polymer-based substrates [7]. Furthermore, structural shrinkage and NP agglomeration during calcination can reduce the effective surface area, thereby affecting the catalytic efficiency of the sensor [54]. The relatively moderate electrical conductivity compared to that of noble metals is another concern. To overcome this problem, additional conductive materials such as graphene [37,40,45], carbon nanotubes (CNTs) [12,48], and noble metals (Au, Pt, Ag) [15,38,62] are often incorporated, increasing the complexity and cost of sensor fabrication. Moreover, many metal oxides require high operating potentials (~0.5–0.8 V vs. Ag/AgCl) [31,42,63], which increases background noise, power consumption, and more importantly the likelihood of side reactions that may interfere with accurate glucose detection [17]. These unintended reactions can generate interfering signals, compromising the accuracy and reliability of glucose detection. Despite these limitations, ongoing research efforts have focused on overcoming these challenges by developing alkaline-free detection systems, improving conductivity through hybrid nanomaterials, and optimizing scalable low-temperature synthesis methods. These advancements will help improve the real-world applicability of metal-oxide-based glucose biosensors, making them more suitable for CGMS, wearable sensors, and point-of-care diagnostics.
4. CONCLUSIONS
The development of glucose biosensors has progressed significantly from enzymatic sensors to highly stable non-enzymatic metal oxide-based platforms. The introduction of metallic oxides, including NiO, CuO, Co3O4, and hybrid composites, has greatly enhanced glucose oxidation efficiency, electron transfer, and sensor stability. These advancements enable higher sensitivity, lower detection limits, and faster response times than those of enzyme-based electrochemical glucose sensors, which are promising candidates for future point-of-care diagnostics and real-time glucose monitoring applications. However, despite these advantages, metal-oxide-based biosensors still face critical challenges, including dependence on strong alkaline electrolytes, high-temperature synthesis requirements, rigid structures, moderate electrical conductivity, and selectivity issues at high overpotentials, which limit their integration into wearable and CGMS devices.
To overcome these limitations, ongoing research is focused on developing alkaline-free glucose-detection systems, enhancing sensor conductivity using hybrid nanomaterials, and designing flexible, low-power biosensors for improved integration into wearable and CGMS devices. The integration of nanostructured materials, AI-driven analytics, and wireless connectivity is expected to drive the development of next-generation non-invasive CGMS systems. With further optimization, metal-oxide-based biosensors have significant potential as affordable, scalable, and highly reliable glucose-sensing technologies in both clinical and personal healthcare applications.
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF-2020R1C1C1005743).
References
-
M. Asif, The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern, J. Educ. Health Promot. 3 (2014) 1.
[https://doi.org/10.4103/2277-9531.127541]
 [https://doi.org/10.1021/acs.iecr.1c03662]
[https://doi.org/10.1021/acs.iecr.1c03662]

-
Y.M. Chitare, S.B. Jadhav, P.N. Pawaskar, V.V. Magdum, J.L. Gunjakar, C.D. Lokhande, Metal Oxide-Based Composites in Nonenzymatic Electrochemical Glucose Sensors, Ind. Eng. Chem. Res. 60 (2021) 18195–18217.
[https://doi.org/10.1021/acs.iecr.1c03662]

-
P. Saeedi, I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, et al., Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition, Diabetes Res. Clin. Pract. 157 (2019) 107843.
[https://doi.org/10.1016/j.diabres.2019.107843]

-
A.D. Deshpande, M. Harris-Hayes, M. Schootman, Epidemiology of diabetes and diabetes-related complications, Phys. Ther. 88 (2008) 1254–1264.
[https://doi.org/10.2522/ptj.20080020]

-
A. Mohammadpour-Haratbar, S. Mohammadpour-Haratbar, Y. Zare, K.Y. Rhee, S.J. Park, A Review on Non-Enzymatic Electrochemical Biosensors of Glucose Using Carbon Nanofiber Nanocomposites, Biosens. (Basel) 12 (2022) 1004.
[https://doi.org/10.3390/bios12111004]

-
G. Wang, X. He, L. Wang, A. Gu, Y. Huang, B. Fang, et al., Non-enzymatic electrochemical sensing of glucose, Microchimica Acta. 180 (2012) 161–186.
[https://doi.org/10.1007/s00604-012-0923-1]

-
M. Safarkhani, A. Aldhaher, G. Heidari, E.N. Zare, M.E. Warkiani, O. Akhavan, et al., Nanomaterial-assisted wearable glucose biosensors for noninvasive real-time monitoring: Pioneering point-of-care and beyond, Nano Mater. Sci. 6 (2024) 263–283.
[https://doi.org/10.1016/j.nanoms.2023.11.009]

-
M.-J. Kim, D.-J. Han, G.-E. Choi, R.-Y. Park, D.-W. Park, DPP-DTT Thin-Film-Transistor-based Glucose Sensor with Parylene C Gate Dielectric, J. Sens. Sci. Technol. 33 (2024) 474–480.
[https://doi.org/10.46670/JSST.2024.33.6.474]

-
Y. Lee, S. Biswas, M. Koo, H. Kim, Recent Development in Biocompatible Biosensors, J. Sens. Sci. Technol. 32 (2023) 403–411.
[https://doi.org/10.46670/JSST.2023.32.6.403]

-
J. Xiao, Y. Liu, L. Su, D. Zhao, L. Zhao, X. Zhang, Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose, Anal Chem. 91 (2019) 14803–14807.
[https://doi.org/10.1021/acs.analchem.9b03110]

-
Y.L. Kim, Y.-M. Kim, J. Oh, J.H. Shin, Copy Paper as a Platform for Low-cost Sensitive Glucose Sensing, J. Sens. Sci. Technol. 32 (2023) 16–21.
[https://doi.org/10.46670/JSST.2023.32.1.16]

-
S. Soylemez, B. Yoon, L. Toppare, T.M. Swager, Quaternized Polymer-Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor, ACS Sens. 2 (2017) 1123–1127.
[https://doi.org/10.1021/acssensors.7b00323]

-
Y.J. Heo, H. Shibata, T. Okitsu, T. Kawanishi, S. Takeuchi, Long-term in vivo glucose monitoring using fluorescent hydrogel fibers, PNAS 108 (2011) 13399–13403.
[https://doi.org/10.1073/pnas.1104954108]

-
S.W. Lee, K.Y. Lee, Y.W. Song, W.K. Choi, J. Chang, H. Yi, Direct Electron Transfer of Enzymes in a Biologically Assembled Conductive Nanomesh Enzyme Platform, Adv. Mater. 28 (2016) 1577–1584.
[https://doi.org/10.1002/adma.201503930]

-
K.V. Jarnda, D.Q. Wang, Qurrat-Ul-Ain, R. Anaman, V.E. Johnson, G.P. Roberts, et al., Recent advances in electrochemical non-enzymatic glucose sensor for the detection of glucose in tears and saliva: A Review, Sens. Actuators A Phys. 363 (2023) 114778.
[https://doi.org/10.1016/j.sna.2023.114778]

-
L. Johnston, G. Wang, K. Hu, C. Qian, G. Liu, Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables, Front Bioeng. Biotechnol. 9 (2021) 733810.
[https://doi.org/10.3389/fbioe.2021.733810]

-
H. Zhu, L. Li, W. Zhou, Z. Shao, X. Chen, Advances in non-enzymatic glucose sensors based on metal oxides, J. Mater. Chem B 4 (2016) 7333–7349.
[https://doi.org/10.1039/C6TB02037B]

-
L.C. Clark, C. Lyons, Electrode Systems for Continuous Monitoring in Cardiovascular Surgery, Ann Ny Acad Sci 102 (1962) 29–45.
[https://doi.org/10.1111/j.1749-6632.1962.tb13623.x]

-
J. Wang, Glucose biosensors: 40 years of advances and challenges, Electroanal. 13 (2001) 983–988.
[https://doi.org/10.1002/1521-4109(200108)13:12<983::AID-ELAN983>3.0.CO;2-#]

-
S. Park, H. Boo, T.D. Chung, Electrochemical non-enzymatic glucose sensors, Anal. Chim. Acta 556 (2006) 46–57.
[https://doi.org/10.1016/j.aca.2005.05.080]

-
S.H. Min, H.Y. Geng, Y.H. He, T.L. Xu, Q.Z. Liu, X.J. Zhang, Minimally and non-invasive glucose monitoring: the road toward commercialization, Sens. Diagn. (2025) 1–27.
[https://doi.org/10.1016/j.aca.2005.05.080]

- J. Pak, W. Chang, C.H. Kwon, J. Cho, Recent Advances in Enzyme-based Biofuel Cells Using Glucose Fuel: Achieving High Power Output and Enhanced Operational Stability, Adv. Funct. Mater. (2024) 2415933.
-
J. Li, H.F. Hu, H.Y. Li, C.B. Yao, Recent developments in electrochemical sensors based on nanomaterials for determining glucose and its byproduct H2O2, J. Mater. Sci. 52 (2017) 10455–10469.
[https://doi.org/10.1002/adfm.202415933]
 [https://doi.org/10.1007/s10853-017-1221-4]
[https://doi.org/10.1007/s10853-017-1221-4]

-
R. Singh, J. Singh, Recent Advances in Nanostructured Cobalt Oxide (Co3O4): Addressing Methods and Design Strategies, Challenges, and Future Directions for Non-Enzymatic Sensor Applications, Sens. Actuators A Phys. (2025) 116404.
[https://doi.org/10.1016/j.sna.2025.116404]

-
D. Pletcher, Electrocatalysis - Present and Future, J. Appl. Electrochem. 14 (1984) 403–415.
[https://doi.org/10.1007/BF00610805]

-
L.D. Burke, Premonolayer Oxidation and Its Role in Electrocatalysis, Electrochim. Acta 39 (1994) 1841–1848.
[https://doi.org/10.1016/0013-4686(94)85173-5]

-
J.J. Liu, X.T. Zha, Y.J. Yang, MOF-based Sensing Materials for Non-enzymatic Glucose Sensors, J. Electrochem. Sci. Technol. 15 (2024) 32–50.
[https://doi.org/10.33961/jecst.2023.00626]

-
Y. Ding, Y. Wang, L. Su, M. Bellagamba, H. Zhang, Y. Lei, Electrospun Co3O4 nanofibers for sensitive and selective glucose detection, Biosens. Bioelectron. 26 (2010) 542–548.
[https://doi.org/10.1016/j.bios.2010.07.050]

-
L. Tian, G. He, Y. Cai, S. Wu, Y. Su, H. Yan, et al., Co3O4 based non-enzymatic glucose sensor with high sensitivity and reliable stability derived from hollow hierarchical architecture, Nanotechnol. 29 (2018) 075502.
[https://doi.org/10.1088/1361-6528/aaa1d2]

-
X. Strakosas, J. Selberg, P. Pansodtee, N. Yonas, P. Manapongpun, M. Teodorescu, et al., A non-enzymatic glucose sensor enabled by bioelectronic pH control, Sci. Rep. 9 (2019) 10844.
[https://doi.org/10.1038/s41598-019-46302-9]

-
Q. Balouch, Z.H. Ibupoto, G.Q. Khaskheli, R.A. Soomro, Sirajuddin, M.K. Samoon, et al., Cobalt Oxide Nanoflowers for Electrochemical Determination of Glucose, J. Electron. Mater. 44 (2015) 3724–3732.
[https://doi.org/10.1007/s11664-015-3860-z]

-
K. Tian, K. Baskaran, A. Tiwari, Nonenzymatic glucose sensing using metal oxides - Comparison of CuO, Co3O4, and NiO, Vacuum 155 (2018) 696–701.
[https://doi.org/10.1016/j.vacuum.2018.06.060]

-
C. Hou, Q. Xu, L. Yin, X. Hu, Metal-organic framework templated synthesis of Co3O4 nanoparticles for direct glucose and H2O2 detection, Analyst 137 (2012) 5803–5808.
[https://doi.org/10.1039/c2an35954e]

-
F. Zadehahmadi, N.T. Eden, H. Mahdavi, K. Konstas, J.I. Mardel, M. Shaibani, et al., Removal of metals from water using MOF-based composite adsorbents, Environ. Sci. Water Res. Technol. 9 (2023) 1305–1330.
[https://doi.org/10.1039/D2EW00941B]

-
P. Mohana, S. Swathi, R. Yuvakkumar, G. Ravi, M. Thambidurai, H.D. Nguyen, Co3O4/g-C3N4 nanocomposite for enriched electrochemical water splitting, Int. J. Hydrogen Energy 49 (2024) 376–389.
[https://doi.org/10.1016/j.ijhydene.2023.08.038]

-
T. Chen, X. Li, C. Qiu, W. Zhu, H. Ma, S. Chen, et al., Electrochemical sensing of glucose by carbon cloth-supported Co3O4/PbO2 core-shell nanorod arrays, Biosens. Bioelectron. 53 (2014) 200–206.
[https://doi.org/10.1016/j.bios.2013.09.059]

-
X.C. Dong, H. Xu, X.W. Wang, Y.X. Huang, M.B. Chan-Park, H. Zhang, et al., 3D graphene-cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection, ACS Nano 6 (2012) 3206–3213.
[https://doi.org/10.1021/nn300097q]

-
X.Y. Lang, H.Y. Fu, C. Hou, G.F. Han, P. Yang, Y.B. Liu, et al., Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors, Nat. Commun. 4 (2013) 2169.
[https://doi.org/10.1038/ncomms3169]

-
R.K. Sahoo, A. Das, K. Samantaray, S.K. Singh, R.S. Mane, H.C. Shin, et al., Electrochemical glucose sensing characteristics of two-dimensional faceted and non-faceted CuO nanoribbons, Crystengcomm 21 (2019) 1607–1616.
[https://doi.org/10.1039/C8CE02033G]

-
X.L. Wang, E.L. Liu, X.L. Zhang, Non-enzymatic glucose biosensor based on copper oxide-reduced graphene oxide nanocomposites synthesized from water-isopropanol solution, Electrochim. Acta 130 (2014) 253–260.
[https://doi.org/10.1016/j.electacta.2014.03.030]

-
H.Z. Zhang, Y.W. Yu, X.D. Shen, X.L. Hu, A Cu2O/Cu/carbon cloth as a binder-free electrode for non-enzymatic glucose sensors with high performance, New J. Chem. 44 (2020) 1993–2000.
[https://doi.org/10.1039/C9NJ05256A]

-
W. Jia, M. Guo, Z. Zheng, T. Yu, Y. Wang, E.G. Rodriguez, et al., Vertically Aligned CuO Nanowires Based Electrode for Amperometric Detection of Hydrogen Peroxide, Electroanalysis 20 (2008) 2153–2157.
[https://doi.org/10.1002/elan.200804299]

-
Y. Cudennec, A. Lecerf, The transformation of Cu(OH)2 into CuO, revisited, Solid State Sci. 5 (2003) 1471–1474.
[https://doi.org/10.1016/j.solidstatesciences.2003.09.009]

-
S. Boakye-Ansah, Y.T. Lim, H.J. Lee, W.S. Choi, Structure-controllable superhydrophobic Cu meshes for effective separation of oils with different viscosities and aqueous pollutant purification, Rsc Adv. 6 (2016) 17642–17650.
[https://doi.org/10.1039/C6RA00346J]

-
Q. Li, D.S. Yang, Y. Gao, Z.Y. Wang, R.X. Yin, F.Z. Xuan, Highly Sensitive and Flexible Copper Oxide/Graphene Non-Enzymatic Glucose Sensor by Laser Direct Writing, Adv. Sens. Res. 2 (2023) 2200067.
[https://doi.org/10.1002/adsr.202200067]

-
Y.M. Luo, Q.Y. Wang, J.H. Li, F. Xu, L.X. Sun, Y.T. Bu, et al., Tunable hierarchical surfaces of CuO derived from metal-organic frameworks for non-enzymatic glucose sensing, Inorg. Chem. Front. 7 (2020) 1512–1525.
[https://doi.org/10.1039/D0QI00104J]

-
M. Fleischmann, K. Korinek, D. Pletcher, The oxidation of organic compounds at a nickel anode in alkaline solution, J. Electroanal. Chem. Interfacial Electrochem. 31 (1971) 39–49.
[https://doi.org/10.1016/S0022-0728(71)80040-2]

-
R. Prasad, N. Gorjizadeh, R. Rajarao, V. Sahajwalla, B.R. Bhat, Plant root nodule like nickel-oxide-multi-walled carbon nanotube composites for non-enzymatic glucose sensors, RSC Adv. 5 (2015) 44792–44799.
[https://doi.org/10.1039/C5RA03720D]

-
S.T. Wang, C. Wang, G.J. Wei, H.Q. Xiao, N. An, Y. Zhou, et al., Non-enzymatic glucose sensor based on facial hydrothermal synthesized NiO nanosheets loaded on glassy carbon electrode, Colloids Surf. A 509 (2016) 252–258.
[https://doi.org/10.1016/j.colsurfa.2016.08.076]

-
G. He, L. Tian, Y. Cai, S. Wu, Y. Su, H. Yan, et al., Sensitive Non-enzymatic Electrochemical Glucose Detection Based on Hollow Porous NiO, Nanoscale Res. Lett. 13 (2018) 1–10.
[https://doi.org/10.1186/s11671-017-2406-0]

-
S. Sedaghat, C.R. Piepenburg, A. Zareei, Z.M. Qi, S. Peana, H.Y. Wang, et al., Laser-Induced Mesoporous Nickel Oxide as a Highly Sensitive Non-enzymatic Glucose Sensor, ACS Appl. Nano Mater. 3 (2020) 5260–5270.
[https://doi.org/10.1021/acsanm.0c00659]

-
X.T. Wang, M.G. Zhao, H. Li, Y.W. Song, Y.F. Cheng, S.G. Chen, Introducing Schottky interface as a novel strategy for ultrasensitive non-enzymatic glucose detection, J. Electroanal. Chem. 801 (2017) 251–257.
[https://doi.org/10.1016/j.jelechem.2017.07.026]

-
S. Kailasa, B.G. Rani, M.S.B. Reddy, N. Jayarambabu, P. Munindra, S. Sharma, K.V. Rao, NiO nanoparticles -decorated conductive polyaniline nanosheets for amperometric glucose biosensor, Mater. Chem. Phys. 242 (2020) 122524.
[https://doi.org/10.1016/j.matchemphys.2019.122524]

-
G. Zeng, W. Li, S. Ci, J. Jia, Z. Wen, Highly Dispersed NiO Nanoparticles Decorating graphene Nanosheets for Nonenzymatic Glucose Sensor and Biofuel Cell, Sci. Rep. 6 (2016) 36454.
[https://doi.org/10.1038/srep36454]

-
Z. Lotfi, M.B. Gholivand, M. Shamsipur, Non-enzymatic glucose sensor based on a g-C3N4/NiO/CuO nanocomposite, Anal. Biochem. 616 (2021) 114062.
[https://doi.org/10.1016/j.ab.2020.114062]

-
V. Archana, Y. Xia, R.Y. Fang, G.G. Kumar, Hierarchical CuO/NiO-Carbon Nanocomposite Derived from Metal Organic Framework on Cello Tape for the Flexible and High Performance Nonenzymatic Electrochemical Glucose Sensors, ACS Sustainable Chem. Eng. 7 (2019) 6707–6719.
[https://doi.org/10.1021/acssuschemeng.8b05980]

-
M. Li, L. Liu, Y. Xiong, X. Liu, A. Nsabimana, X. Bo, L. Guo, Bimetallic MCo (M=Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection, , Sens. Actuators B Chem. 207 (2015) 614-622.
[https://doi.org/10.1016/j.snb.2014.10.092]

-
S.Y. Cheng, S. DelaCruz, C. Chen, Z.R. Tang, T.L. Shi, C. Carraro, R. Maboudian, Hierarchical Co3O4/CuO nanorod array supported on carbon cloth for highly sensitive nonenzymatic glucose biosensing, Sens. Actuators B Chem. 298 (2019) 126860.
[https://doi.org/10.1016/j.snb.2019.126860]

-
B. Xue, K.Z. Li, L. Feng, J.H. Lu, L.L. Zhang, Graphene wrapped porous Co3O4/NiCo2O4 double-shelled nanocages with enhanced electrocatalytic performance for glucose sensor, Electrochim. Acta 239 (2017) 36–44.
[https://doi.org/10.1016/j.electacta.2017.04.005]

-
C. Guati, L. Gomez-Coma, M. Fallanza, I. Ortiz, Progress on the influence of non-enzymatic electrodes characteristics on the response to glucose detection: a review (2016–2022), Rev. Chem. Eng. 40 (2024) 123–148.
[https://doi.org/10.1515/revce-2022-0058]

-
H. Abunahla, B. Mohammad, A. Alazzam, M.A. Jaoude, M. Al-Qutayri, S. Abdul Hadi, et al., MOMSense: Metal-Oxide-Metal Elementary Glucose Sensor, Sci. Rep. 9 (2019) 5524.
[https://doi.org/10.1038/s41598-019-41892-w]

-
A.K. Mishra, B. Mukherjee, A. Kumar, D.K. Jarwal, S. Ratan, C. Kumar, et al., Superficial fabrication of gold nanoparticles modified CuO nanowires electrode for nonenzymatic glucose detection, RSC Adv. 9 (2019) 1772–1781.
[https://doi.org/10.1039/C8RA07516F]

-
Y. Ding, Y. Liu, J. Parisi, L. Zhang, Y. Lei, A novel NiO-Au hybrid nanobelts based sensor for sensitive and selective glucose detection, Biosens. Bioelectron. 28 (2011) 393–398.
[https://doi.org/10.1016/j.bios.2011.07.054]


Seung-Woo Lee is an Associate Professor in the Department of Fine Chemistry at Seoul National University of Science and Technology (SEOULTECH). He earned his B.E. degree in Fine Chemical Engineering and Applied Chemistry from Chungnam National University in 2006 and completed his Ph.D. in Chemical and Biological Engineering at the University of Nebraska-Lincoln in 2013. Following his doctoral studies, he received postdoctoral training at the Post-Silicon Semiconductor Institute, Korea Institute of Science and Technology (KIST). His research interests focus on nano-bio hybrid materials, electrochemical biosensing and chemical sensing, electrochemical energy devices, and functional materials for environmental applications.