Emerging Machine Learning in Wearable Healthcare Sensors
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License(https://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Human biosignals provide essential information for diagnosing diseases such as dementia and Parkinson's disease. Owing to the shortcomings of current clinical assessments, noninvasive solutions are required. Machine learning (ML) on wearable sensor data is a promising method for the real-time monitoring and early detection of abnormalities. ML facilitates disease identification, severity measurement, and remote rehabilitation by providing continuous feedback. In the context of wearable sensor technology, ML involves training on observed data for tasks such as classification and regression with applications in clinical metrics. Although supervised ML presents challenges in clinical settings, unsupervised learning, which focuses on tasks such as cluster identification and anomaly detection, has emerged as a useful alternative. This review examines and discusses a variety of ML algorithms such as Support Vector Machines (SVM), Random Forests (RF), Decision Trees (DT), Neural Networks (NN), and Deep Learning for the analysis of complex clinical data.
Keywords:
Biosignals, Wearable sensors, Machine learning in healthcare, Parkinson's disease detection, Clinical data analysis, ML algorithms in medicine1. INTRODUCTION
Biological signals, also referred to as biosignals originating from the human body, carry vital information regarding the physiological state of the body. This information is useful for assessing a range of illnesses, functional difficulties, and cognitive impairments such as Parkinson's disease, dementia, irregular walking patterns, multiple sclerosis, rapid eye movement (REM) sleep disorders, and various neurological disorders [1-3]. Early detection of these medical conditions is crucial for prompt diagnosis and implementation of specific treatments to prevent them from becoming life-threatening. Fig. 1 illustrates the visualization of biosignals in humans along with their corresponding measurements.
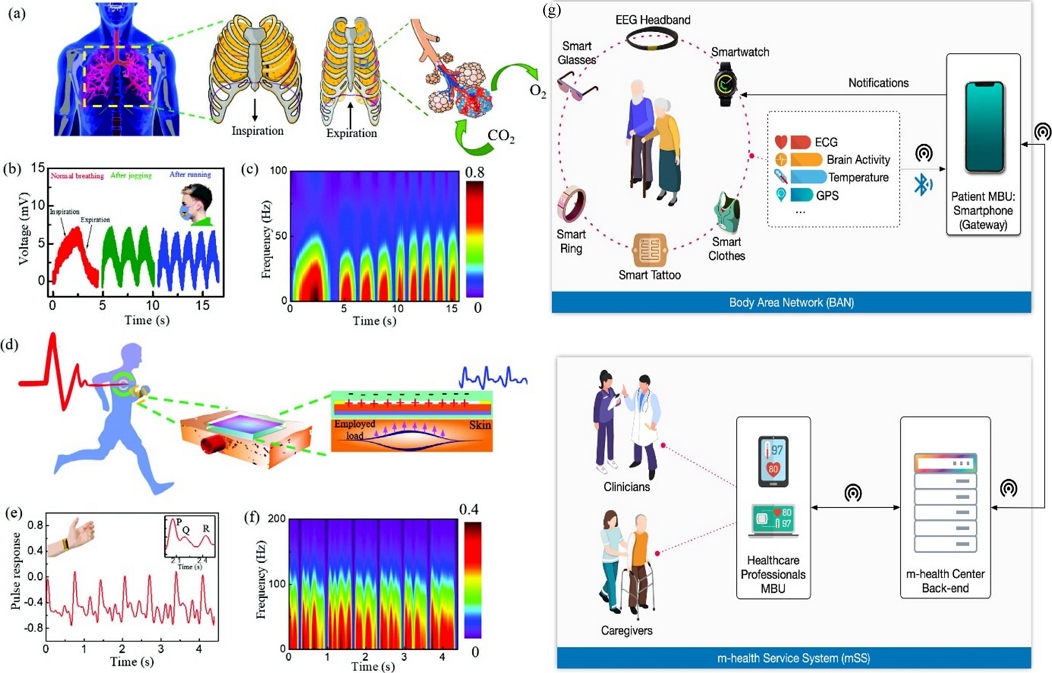
Several biosignals in humans and their measurements. (a) Schematic of the human breathing mechanism, (b) human breathing measurements at different breathing conditions by Wearable Triboelectric Nanogenerator (W-TENG), (c) the frequency of the breathing with time is plotted using Short Term Fourier Transform (STFT) at the different breathing rates. Another example is shown in (d) for monitoring of arterial pulse by W-TENG, (e) arterial pulse monitoring by W-TENG, and (f) the STFT plot visualization. For elderly health monitoring condition, it can be done using (g) a generic mobile health (m-health) using smartphone and set of sensors. Fig. (a) to (f) reprinted with permission from Ref. [1]. Copyright (2023) John Wiley and Sons, Inc. and Fig. (g) reprinted with permission from Ref. [3]. Copyright (2022) Elsevier Ireland LTD.
Although an early and accurate diagnosis is vital, most clinical assessments conducted today rely on direct observations and patient self-reports [4,5], which are susceptible to subjectivity and lack practical monitoring tools. These limitations must be overcome to develop a noninvasive and accurate method for collecting patient data, assessing disease states, evaluating the impact of medical interventions, and continuously monitoring patient conditions in actual settings [3]. Such clinical tools can be developed using machine learning (ML) techniques. ML algorithms have been applied to wearable sensor data in healthcare in three primary areas: (1) detecting abnormal movements, (2) identifying diseases, and (3) assisting in rehabilitation and therapy [6].
Critical information on the severity of disorders such as Essential Tremor (ET), Parkinson's disease (PD), and osteoarthritis in the knees can be obtained from irregularities in bodily movements [1,7-10]. As another example, in the case of autism [11], children’s learning of new skills and the use of acquired skills are negatively affected by abnormal body movements. In such cases, it is critical to identify patients' locomotion impairments to enhance their quality of life. By applying ML to wearable sensors, these abnormalities can be automatically detected in real time. This can be achieved using a classifier to create a general decision boundary that separates abnormal movement classes from normal movement classes in high-dimensional input data. Alternatively, the distribution of normal movements can be observed using anomaly detection techniques, and any significant deviation from this distribution can be classified as a movement anomaly.
Disease identification involves assessing disease severity, which can be represented by clinical scores [12]. For instance, the assessment of atypical motor movements can be used to gauge disease severity in patients with Parkinson's disease who suffer from motor disturbances. In existing literature, ML algorithms, including classification and regression models, are predominantly used on wearable sensor data to measure disease severity and body abnormalities [7,9,13-15].
Rehabilitation is an effective means of improving functionality and reducing disability in individuals with illnesses. Therapy sessions in clinical settings can be long, expensive, and impractical. Therefore, developing a remotely operable system for home-based rehabilitation is necessary [6]. This system can built using wearable sensor technology, enabling real-time data collection. With such a system, patients can receive real-time feedback and rehabilitation can be continuously monitored. ML algorithms provide the possibility of measuring indicators of rehabilitation progress and identifying patient activities [16].
2. MACHINE LEARNING ON WEARABLE SENSORS
ML represents a subset of Artificial Intelligence (AI) that provides computational tools for parameter learning based on observed data, referred to as training data. As they relate to wearable sensor technologies, these input data usually comprise multichannel time-series data that record movements and/or physiological signals from body-attached sensors. During the learning process, an objective function specific to a task is optimized to minimize or maximize the performance metrics. Task definition varies depending on its intended use; for instance, it may involve regression to predict continuous clinical metrics, such as symptom severity or physical/cognitive loads, or classification to identify categorical body states in patients [17,18]. The process of predicting the unobserved data using the learned parameters of a model is called inference.
Numerous studies have focused on the application of supervised learning to wearable sensor data analysis [19-21]. However, supervised ML algorithms has a major disadvantage, especially when used in clinical contexts, because they rely on labeled data. Labeling data is time-consuming, costly, and subjective in clinical environments. This drawback highlights the importance of unsupervised learning, which is located at the opposite ends of the ML spectrum. In unsupervised learning, tasks such as cluster identification or anomaly detection within the input data are the primary focus, and no labeled data are required. For example, clustering can be used to divide a population into separate categories based on the wearable sensor-recorded daily activity patterns [20]. In anomaly detection scenarios [22], the objective is to identify abnormal patterns in a data signal.
The following sections describe the various ML techniques frequently used to analyze data from wearable sensors. These include Support Vector Machines (SVM), Random Forests (RF), Decision Trees (DT), Neural Networks (NN), and Deep Learning [23]. Their benefits and limitations in the analysis of complicated data in clinical settings are also discussed.
2.1. SUPPORT VECTOR MACHINES (SVM)
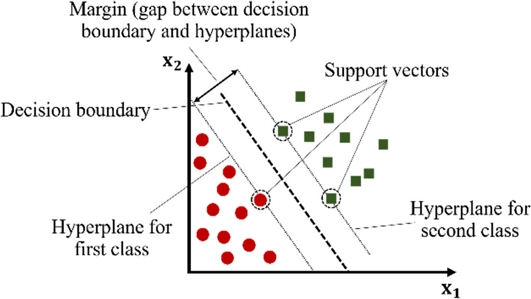
One type of ML technique used for binary classification problems is the Support Vector Machines (SVM). SVM works on the fundamental principle of locating a hyperplane in a multidimensional space that divides data into two classes. In essence, a hyperplane is a decision boundary that separates data points based on their classification. This hyperplane is a line in two dimensions; however, it may be a plane or a higher-dimensional surface. The goal of the SVM algorithm is to determine the optimal hyperplane with the maximum margin, which is the maximum distance between the data points of both classes [24]. It is accomplished by limiting the use of support vectors, which are a subset of the training samples. The data points in question were the closest to the decision border. The orientation of the hyperplane was determined by the position of the support vectors. When data cannot be separated linearly, SVM employs a technique known as the kernel trick. This process involves converting the input data into a higher-dimensional space, such that the data points can be divided using a hyperplane. Using a linear hyperplane, an SVM can separate nonlinearly separable support vectors by selecting a kernel function that considers the data characteristics [25]. SVM is memory-efficient and particularly useful in high-dimensional domains. However, it is susceptible to the choice of kernel parameters and do not offer probability estimation [6]. The SVM visualization is shown in Fig. 2.
SVM plays a crucial role in the analysis of wearable sensor data in the medical field. One application involves assessing symptom severity in patients with conditions, such as Parkinson’s disease, in which SVM gauges abnormal motor movement intensity. In addition, SVM can recognize the activities of patients and measure indicators of rehabilitation progress [6].
However, selecting a particular application relies on the nature of the data and the prevailing problems. For example, SVM can improve the clinical setting performance of wearable medical devices by obtaining signal features specific to the needs of older patients, SVM could improve compressive sensing performance in wearable medical devices [26]. Although supervised ML algorithms, such as SVM, hold great potential, it is crucial to recognize that they require labeled data. This presents a barrier in clinical settings, because data labeling is subjective, expensive, and time-consuming.
2.2. RANDOM FORESTS (RF) AND DECISION TREES (DT)
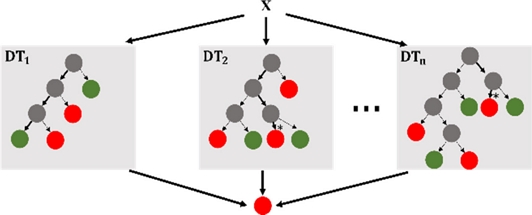
Random Forests (RF) and Decision Trees (DT) are frequently employed [6]. A supervised ML approach called DT divides the input space into subgroups by employing a structure such as a tree. Starting at the root node, it progresses via internal nodes and leaves based on the attribute-based decision functions. These functions guide samples through a tree during testing to predict the class labels [6]. Using an ensemble approach called RF, multiple DTs were constructed and their respective class modes were produced. RF is well suited for systems with limited computational resources owing to its excellent accuracy and short training time [32]. In the field of medicine, these algorithms help people manage health problems in Internet of Things (IoT) healthcare applications [27] and diagnose illnesses such as breast cancer more effectively [18].
In contrast, quantization optimization methods, such as Tiny Transfer Learning (TinyTL), can drastically lower the training memory footprint for efficient on-device learning. To evaluate the model performance, more research is required that considers accuracy, latency, and power consumption [32]. Fig. 3 shows RF and DT visualizations.
2.3. NEURAL NETWORKS (NN)
A feedforward neural network, also known as a multilayer perceptron (MLP), operates in a hierarchical architecture with layers of processing nodes. Using weighted links, each layer establishes connections with the layers below it. To transfer information from one layer to the next, the neurons in each layer perform a weighted linear summation followed by an activation function, such as a sigmoid or tangent hyperbolic. The final layer, referred to as the output layer, takes inputs from the layers before transforming them into output values [6]. Network weights were modified throughout the training phase using a backpropagation technique. In the forward stage of backpropagation, the network uses randomly initialized weights to calculate the outputs for the given inputs. Subsequently, by utilizing the chain rule of partial derivatives, the errors traverse backward through the network to fine-tune the interlayer weights when the projected and actual outputs are compared.
Several studies have reported that Neural Networks (NN) can be used to analyze wearable sensor data. For instance, one study utilized an NN model to forecast disease states in patients with PD based on accelerometer data gathered in both the home and laboratory environments [28]. Another study applied an NN model to the time- and frequency-domain features extracted from physiological and accelerometer sensors to identify the daily activities of healthy subjects outside the laboratory [29].
Owing to their effectiveness in learning intricate and nonlinear functions, NN's computational efficiency of NN during testing renders them suitable for real-time clinical applications. However, their susceptibility to overfitting is a drawback. The illustration of CNN as another type of NN is shown in Fig. 4.
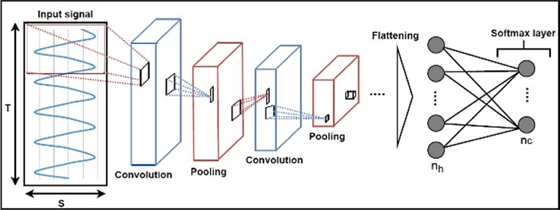
Illustration of a Convolutional Neural Network (CNN) model with convolutional layers, pooling layers, h dense layers, and c output classes represented by a softmax layer. Input data are processed by convolutional layers and pooling layers and are passed to dense layers after extraction of profound features reprinted with permission from Ref. [18]. Copyright (2022) MDPI.
In Fig. 4, the convolutional layers serve as the primary components typically employed to execute convolutional operations between one or multiple convolutional filters (or kernels) learned during the training phase and the layer input. This convolution operation is conducted by sliding the convolution kernels over the input data. In [18], raw sensor data is presented as 3D input (S × T × 1) to the CNN model for processing. Following a sequence of convolutional and pooling layers, the output of the final convolutional layer is typically transformed into a smoothed 1D vector and then directed into the softmax layer. The Rectified Linear Unit (ReLU) stands as the most frequently used activation function for convolutional layers. Additionally, it's common to incorporate multiple dense layers of a multilayer perceptron into the CNN architecture to address classification problems. In such scenarios, a softmax activation function is generally employed to connect the last dense layer to the output layer.
2.4. DEEP LEARNING
Deep Learning is a branch of ML that uses multi-layered neural networks to analyze and generate predictions or decisions based on a variety of inputs. Its effectiveness is particularly evident when handling high-dimensional data, making it suitable for the analysis of wearable sensor data [30]. Deep learning is used in healthcare applications to analyze the large and complex datasets generated by devices. For instance, deep learning systems can examine data from accelerometers, heart rate monitors, and other sensors to identify patterns that may indicate illnesses or other health problems [6]. An example is the analysis of time series data from wearable sensors using CNN. CNN, which are categorized as deep learning models, excel at analyzing spatial data, making them suitable for tasks such as recognizing activities based on accelerometer data [30].
Wearable sensor data processing is further aided by another class of deep learning models called Recurrent Neural Networks (RNN). RNN is optimized for sequential data and are therefore well-suited for tasks such as assessing an accelerometer reading or a sequence of heart rate measurements [6].
Although deep learning can yield insightful information from wearable sensor data, it has certain drawbacks. Wearables with limited processing power and battery life may find it difficult to train deep-learning models because they can be computationally demanding and require large amounts of data [27].
3. WEARABLE SENSOR DATA ANALYSIS USING MACHINE LEARNING
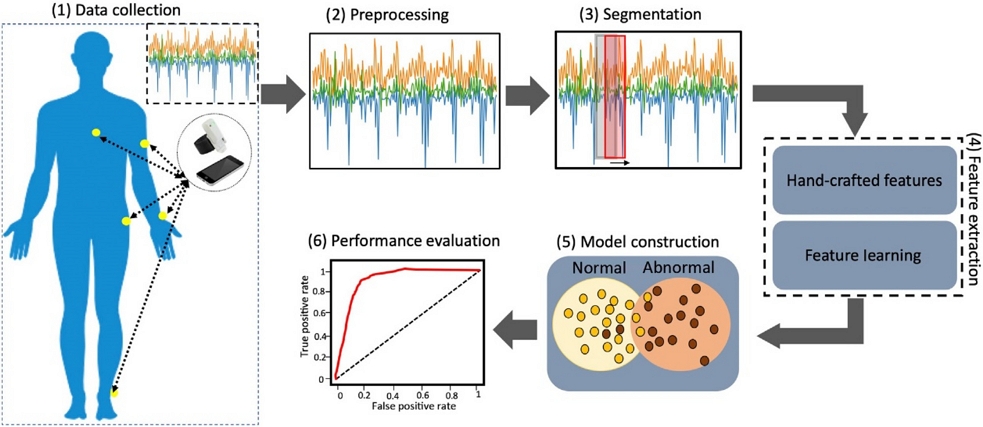
The analysis of the wearable sensor data followed a structured six-step pipeline. Initially, data are gathered from wearable sensors, with the specific type contingent on the sensor and observed health condition. For example, a motion sensor records movement data, whereas a heart rate monitor records heart rate data. Subsequently, the collected data were preprocessed to eliminate errors or inconsistencies, normalize the values, and transform them into formats compatible with ML algorithms. The third step involved segmenting the data into time-specific windows, each containing a set of data points. Following segmentation, features are extracted from each data segment to represent measurable characteristics that distinguish various health conditions or states. Once the features are known, a suitable algorithm is selected and trained on the retrieved data to create a machine learning model. The goal of the model was to use attributes to learn, predict, or classify health problems. The pipeline ends with an assessment of the performance of the ML model using a different test set to measure the recall, accuracy, precision, and F1 score, which are determined by the following formulas [6]:
| (1) |
| (2) |
| (3) |
where tp denotes the true positive, fp denotes the false positive, and fn denotes the false negative for all entries. True positive (tp) refers to the accurate identification of abnormal samples, whereas false positive (fp) denotes instances where normal samples are inaccurately classified as abnormal movements. False negatives correspond to samples incorrectly identified as exhibiting normal movement. [18]. These systematic approaches are illustrated in Fig. 5.
4. CHALLENGES AND OPPORTUNITIES
ML faces various challenges in wearable sensor devices and healthcare systems. Systems for Human Activity Recognition (HAR) have many challenges when dealing with various input features such as different activities and individual traits [31]. Although implementing models on wearable devices enhances privacy, integration challenges arise from limited computing power, storage, and battery life [32]. Sensor data noise increases the complexity, necessitating a careful balance between retaining predictive power and avoiding measurement noise [33]. If the first training fails to capture a wide range of situations, then lightweight algorithms that depend on aligned training data exhibit reduced accuracy [34].
The rapid increase in popularity and theoretical gaps in deep learning have attracted criticism despite its success, further complicating the field of ML applications [31]. Data security and privacy in healthcare have been severely challenged by the focus on on-device data. On-device processing limitations are addressed by methods such as model compression; however, putting them into practice without compromising accuracy is a difficult task. The need for efficient implementation impedes the promise of transfer learning, which uses pretrained models for one task and retrains specific layers for another. This demonstrates how complex the problems ML applications in wearable sensors and healthcare face are [32].
Amidst these challenges, summarizing the previous sections, ML applications in wearable sensor devices and healthcare present several opportunities for advancement.
1) Advanced tools for predictive outcomes: ML algorithms have developed into advanced tools that provide system intelligence for learning, development, and outcome prediction without requiring human intervention. They are widely used in most industries, including cyber security, smart city planning, and financial markets [1].
2) Real-time data analysis: Wearable sensor data can be processed and analyzed by ML models in real time, allowing for assessments and predictions of reserve or decompensation status, as well as activity and context. The user can then receive the summarized data [14].
3) Exer-gaming and physical activity: Children can be encouraged to engage in physical activity using ML in exergaming. A game may become more engaging, motivating, and enhance motor abilities if ML-based feedback is incorporated [16].
4) Identification of symptoms: ML can help recognize the signs of neurological illnesses or movement problems, which can help with diagnosis or symptom treatment [16].
5) Improved healthcare services: ML is essential for the operation of healthcare technologies. Allotting time, completing healthcare requirements, and automating hospital logistics can increase productivity. Algorithms that match the accessibility of medical professionals with appropriate clinical skillsets in the surrounding area can be developed using ML [20].
6) Diagnosis and treatment planning: High-risk patient case detection, prevention, diagnosis, and treatment planning can be aided by ML. By offering an intelligent model that will help physicians and clinicians arrive at the most accurate diagnosis, the diagnostic process [20].
7) Human activity monitoring: ML techniques have been extensively and effectively applied to elderly healthcare systems and the monitoring of human activity. Instead of using manually created feature extraction processes, they automatically extracted features from raw data [31].
5. CONCLUSION AND FUTURE DIRECTION
Transformative breakthroughs in healthcare and wearable sensor technologies can be achieved by incorporating ML technology. Despite the existing obstacles, including diverse input characteristics and privacy issues, ML presents unprecedented possibilities for precise illness identification, real-time monitoring, and personalized treatment strategies. The proposed applications, ranging from human activity monitoring to advanced prediction algorithms, highlight the potential for transforming healthcare procedures and enhancing patient outcomes. Collaborative, multidisciplinary research is essential as we negotiate these obstacles and ensure that upcoming advancements in ML lead to a more efficient, patient-centered healthcare environment.
Improvements in ML for wearable medical devices can improve the well-being of patients. Future research should focus on developing user-friendly HAR systems that can be seamlessly incorporated into daily life, handle various input sources, and encourage long-term adherence. The practicality of continuous monitoring can be improved by workable solutions that optimize ML on wearables while considering battery life and processing power. Innovations should focus on real-time applications such as user-friendly interfaces that provide easily understandable information. ML in remote rehabilitation systems for various situations should be a subject of future research. Bridging theoretical advancements with practical applications is crucial for maximizing ML's potential in personalized and accessible patient care.
Acknowledgments
This study was supported by the following grants: (1) National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT, No. 2021R1A2C3008742) and (2) Technology Innovation Program (02220146) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea).
REFERENCES
-
A. Babu, S. Ranpariya, D. K. Sinha, and D. Mandal, “Deep Learning Enabled Perceptive Wearable Sensor: An Interactive Gadget for Tracking Movement Disorder”, Adv. Mater. Technol., Vol. 8, No. 14, p. 2300046, 2023.
[https://doi.org/10.1002/admt.202300046]

-
B. W. Fling, C. Curtze, and F. B. Horak, “Gait Asymmetry in People with Parkinson’s Disease Is Linked to Reduced Integrity of Callosal Sensorimotor Regions”, Front. Neurol., Vol. 9, pp. 215(1)-215(8), 2018.
[https://doi.org/10.3389/fneur.2018.00215]

-
F. M. Garcia-Moreno, M. Bermudez-Edo, E. Rodríguez-García, J. M. Pérez-Mármol, J. L. Garrido, and M. J. Rodríguez-Fórtiz, “A Machine Learning Approach for Semi-Automatic Assessment of IADL Dependence in Older Adults with Wearable Sensors”, Int. J. Med. Inform., Vol. 157, p. 104625, 2022.
[https://doi.org/10.1016/j.ijmedinf.2021.104625]

-
H. B. Fromme, R. Karani, and S. M Downing, “Direct Observation in Medical Education: A Review of Literature and Evidence for Validity”, Mt. Sinai J. Med., Vol. 76, No. 4, 365-371, 2009.
[https://doi.org/10.1002/msj.20123]

-
A. Neubert, Ó. B. Fernandes, A. Lucevic, M. Pavlova, L. Gulácsi, P. Baji, N. Klazinga, and D. Kringos, “Understanding the Use of Patient-Reported Data by Health Care Insurers: A Scoping Review”, PLoS ONE, Vol. 15, No. 12, p. e0244546, 2020.
[https://doi.org/10.1371/journal.pone.0244546]

-
N. M. Rad and E. Marchiori, “Learning for Healthcare Using Wearable Sensors”, Digital Health, pp 137-149, 2021.
[https://doi.org/10.1016/B978-0-12-818914-6.00007-7]

-
F. C. G. Di Zubiena, G. Menna, I. Mileti, A. Zampogna, F. Asci, M. Paoloni, A. Suppa, Z. Del Prete, and E. Palermo, “Machine Learning and Wearable Sensors for the Early Detection of Balance Disorders in Parkinson’s Disease”, Sensors, Vol. 22, No. 24, pp. 9903(1)-9903(16), 2022.
[https://doi.org/10.3390/s22249903]

-
A. Talitckii, A. Anikina, E. Kovalenko, A. Shcherbak, O. Mayora, O. Zimniakova, E. Bril, M. Semenov, D. V. Dylov, and A. Somov, “Defining Optimal Exercises for Efficient Detection of Parkinson’s Disease Using Machine Learning and Wearable Sensors”, IEEE Trans. Instrum. Meas., Vol. 70, pp. 1-10, 2021.
[https://doi.org/10.1109/TIM.2021.3097857]

-
C. Ma, D. Li, L. Pan, X. Li, C. Yin, A. Li, Z. Zhang, and R. Zong, “Quantitative Assessment of Essential Tremor Based on Machine Learning Methods Using Wearable Device”, Biomed. Signal Process. Control, Vol. 71, p. 103244, 2022.
[https://doi.org/10.1016/j.bspc.2021.103244]

-
J.-S. Tan, S. Tippaya, T. Binnie, P. Davey, K. Napier, J. P. Caneiro, P. Kent, A. Smith, P. O’Sullivan, and A. Campbell, “Predicting Knee Joint Kinematics from Wearable Sensor Data in People with Knee Osteoarthritis and Clinical Considerations for Future Machine Learning Models”, Sensors, Vol. 22, No. 2, pp. 446(1)-446(16), 2022.
[https://doi.org/10.3390/s22020446]

-
U. A. Siddiqui, F. Ullah, A. Iqbal, A. Khan, R. Ullah, S. Paracha, H. Shahzad, and K. S. Kwak, “Wearable-Sensors-Based Platform for Gesture Recognition of Autism Spectrum Disorder Children Using Machine Learning Algorithms”, Sensors, Vol. 21, No. 10, pp. 3319(1)-3319(22), 2021,
[https://doi.org/10.3390/s21103319]

-
T. Mullick, A. Radovic, S. Shaaban, and A. Doryab, “Predicting Depression in Adolescents Using Mobile and Wearable Sensors: Multimodal Machine Learning–Based Exploratory Study”, JMIR Form. Res., Vol. 6, No. 6, p. e35807, 2022.
[https://doi.org/10.2196/35807]

-
A. Ahmed, J. Ramesh, S. Ganguly, R. Aburukba, A. Sagahyroon, and F. Aloul, “Investigating the Feasibility of Assessing Depression Severity and Valence-Arousal with Wearable Sensors Using Discrete Wavelet Transforms and Machine Learning”, Information, Vol. 13, No. 9, pp. 406(1)-406(11), 2022.
[https://doi.org/10.3390/info13090406]

-
J. P. Kimball, O. T. Inan, V. A. Convertino, S. Cardin, and M. N. Sawka, “Wearable Sensors and Machine Learning for Hypovolemia Problems in Occupational, Military and Sports Medicine: Physiological Basis, Hardware and Algorithms”, Sensors, Vol. 22, No. 2, pp. 442(1)-442(25), 2022.
[https://doi.org/10.3390/s22020442]

-
M. K. O’Brien, O. K. Botonis, E. Larkin, J. Carpenter, B. Martin-Harris, R. Maronati, K. Lee, L. R. Cherney, B. Hutchison, S. Xu, J. A. Rogers, and A. Jayaraman, “Advanced Machine Learning Tools to Monitor Biomarkers of Dysphagia: A Wearable Sensor Proof-of-Concept Study”, Digit. Biomark., Vol. 5, No. 2, pp. 167-175, 2021.
[https://doi.org/10.1159/000517144]

-
G. Csizmadia, K. Liszkai-Peres, B. Ferdinandy, Á. Miklósi, and V. Konok, “Human Activity Recognition of Children with Wearable Devices Using LightGBM Machine Learning”, Sci. Rep., Vol. 12, No. 1, pp. 5472(1)-5472(10), 2022.
[https://doi.org/10.1038/s41598-022-09521-1]

-
E. M. Bochniewicz, G. Emmer, A. W. Dromerick, J. Barth, and P. S. Lum, “Measurement of Functional Use in Upper Extremity Prosthetic Devices Using Wearable Sensors and Machine Learning”, Sensors, Vol. 23, No. 6, pp. 3111(1)-3111(13), 2023.
[https://doi.org/10.3390/s23063111]

-
M. T. Irshad, M. A. Nisar, X. Huang, J. Hartz, O. Flak, F. Li, P. Gouverneur, A. Piet, K. M. Oltmanns, and M. Grzegorzek, “SenseHunger: Machine Learning Approach to Hunger Detection Using Wearable Sensors”, Sensors, Vol. 22, No. 20, pp.7711(1)-7711(21), 2022.
[https://doi.org/10.3390/s22207711]

-
D. Ravenscroft, I. Prattis, T. Kandukuri, Y. A. Samad, G. Mallia, and L. G. Occhipinti, “Machine Learning Methods for Automatic Silent Speech Recognition Using a Wearable Graphene Strain Gauge Sensor”, Sensors, Vol. 22, No. 1, pp. 299(1)-299(13), 2021.
[https://doi.org/10.3390/s22010299]

-
A. Kadu, M. Singh, and K. Ogudo, “Novel Scheme for Classification of Epilepsy Using Machine Learning and a Fuzzy Inference System Based on Wearable-Sensor Health Parameters”, Sustainability, Vol. 14, No. 22, pp. 15079(1)-15079(20), 2022.
[https://doi.org/10.3390/su142215079]

-
F. Delmastro, F. Di Martino, and C. Dolciotti, “Cognitive Training and Stress Detection in MCI Frail Older People Through Wearable Sensors and Machine Learning”, IEEE Access, Vol. 8, pp. 65573-65590, 2020.
[https://doi.org/10.1109/ACCESS.2020.2985301]

-
W. K. Lim , S. Davila, J. X. Teo, C. Yang, C. J. Pua, C. Blöcker, J. Q. Lim, J. Ching, J. J. L. Yap, S. Y. Tan, A. Sahlén, C. W.-L. Chin, B. T. Teh, S. G. Rozen, S. A. Cook, K. K. Yeo, and P. Tan, “Beyond Fitness Tracking: The Use of Consumer-Grade Wearable Data from Normal Volunteers in Cardiovascular and Lipidomics Research”, PLoS Biol., Vol. 16, No. 2, pp. e2004285(1)-e2004285(18), 2018.
[https://doi.org/10.1371/journal.pbio.2004285]

-
V. K. Verma and S. Verma, “Machine Learning Applications in Healthcare Sector: An Overview”, Mater. Today. Proc., Vol. 57, pp. 2144-2147, 2022.
[https://doi.org/10.1016/j.matpr.2021.12.101]

- D. Boswell, Introduction to support vector machines, Departement of Computer Science and Engineering University of California, San Diego, 11, pp. 1-15, 2002.
-
K. El Bouchefry and R. S. de Souza, “Learning in Big Data: Introduction to Machine Learning”, In Knowledge Discovery in Big Data from Astronomy and Earth Observation, P. Škoda and F. Adam, Eds. Elsevier, Amsterdam, pp. 225-249, 2020.
[https://doi.org/10.1016/B978-0-12-819154-5.00023-0]

-
T. Ba, S. Li, and Y. Wei, “Data-Driven Machine Learning Integrated Wearable Medical Sensor Framework for Elderly Care Service”, Measurement, Vol. 167, p. 108383, 2021.
[https://doi.org/10.1016/j.measurement.2020.108383]

-
D. K. Jain, K. Srinivas, S. V. N. Srinivasu, and R. Manikandan, “Machine Learning-Based Monitoring System with IoT Using Wearable Sensors and Pre-Convoluted Fast Recurrent Neural Networks (P-FRNN)”, IEEE Sens. J., Vol. 21, No. 22, pp. 25517-25524, 2021.
[https://doi.org/10.1109/JSEN.2021.3091626]

-
J. M. Fisher, N. Y. Hammerla, T. Ploetz, P. Andras, L. Rochester, and R. W. Walker, “Unsupervised Home Monitoring of Parkinson’s Disease Motor Symptoms Using Body-Worn Accelerometers”, Parkinsonism. Relat. Disord., Vol. 33, pp. 44-50, 2016.
[https://doi.org/10.1016/j.parkreldis.2016.09.009]

-
J. Parkka, M. Ermes, P. Korpipaa, J. Mantyjarvi, J. Peltola, and I. Korhonen, “Activity Classification Using Realistic Data from Wearable Sensors”, IEEE Trans. Inform. Technol. Biomed., Vol. 10, No. 1, pp. 119-128, 2006.
[https://doi.org/10.1109/TITB.2005.856863]

-
Y. Yoshida and E. Yuda, “Workout Detection by Wearable Device Data Using Machine Learning”, Appl. Sci., Vol. 13, No. 7, pp. 4280(1)-4280(8), 2023.
[https://doi.org/10.3390/app13074280]

-
J. Muangprathub, A. Sriwichian, A. Wanichsombat, S. Kajornkasirat, P. Nillaor, and V. Boonjing, “Novel Elderly Tracking System Using Machine Learning to Classify Signals from Mobile and Wearable Sensors”, Int. J. Environ. Res. Public Health, Vol. 18, No. 23, pp. 12652(1)-12652(19), 2021.
[https://doi.org/10.3390/ijerph182312652]

-
F. Sabry, T. Eltaras, W. Labda, F. Hamza, K. Alzoubi, and Q. Malluhi, “On-Device Dehydration Monitoring Using Machine Learning from Wearable Device’s Data”, Sensors, Vol. 22, No. 5, pp. 1887(1)-1887(20), 2022.
[https://doi.org/10.3390/s22051887]

-
J. P. Cascales, D. A. Greenfield, E. Roussakis, L. Witthauer, X. Li, A. Goss, and C. L. Evans, “Wireless Wearable Sensor Paired with Machine Learning for the Quantification of Tissue Oxygenation”, IEEE Internet. Things J., Vol. 8, No. 24, pp. 17557-17567, 2021.
[https://doi.org/10.1109/JIOT.2021.3081044]

-
A. Moin, A. Zhou, A. Rahimi, A. Menon, S. Benatti, G. Alexandrov, S. Tamakloe, J. Ting, N. Yamamoto, Y. Khan, F. Burghardt, L. Benini, A. C. Arias, and J. M. Rabaey, “Wearable Biosensing System with In-Sensor Adaptive Machine Learning for Hand Gesture Recognition”, Nat. Electron., Vol. 4, No. 1, pp. 54-63, 2020.
[https://doi.org/10.1038/s41928-020-00510-8]